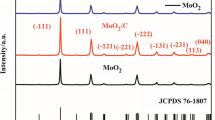
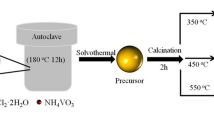
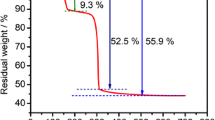
Abstract
Undoubtedly, it is imperative to figure out two stubborn issues concerning low electronic conductivity and sluggish lithium ion diffusion to promote the practical application of Li2FeSiO4 materials in lithium-ion battery (LIB) cathode. Herein, we report an innovative and simple strategy that combines a hydrothermal process with subsequent annealing to synthesize highly uniform Li2FeSiO4/C hollow nanospheres. During the hydrothermal process, polystyrene nanospheres are employed not only as the template but also, more tactfully, as carbon source to form amorphous carbon layers, which will function to enhance the electronic conductivity and restrict particle aggregations. The use of the LIB Li2FeSiO4/C hollow nanospheres as a LIB cathode delivers a desired stable capacity at each rate stage, and even at a high rate of 10 C, the hollow nanosphere cathode can present a specific discharge capacity as high as 50.5 mAh·g−1. After 100 cycles, the capacity retentions at 1 and 10 C remain as high as 93% and 72%, respectively. The superior electrochemical performance is believed to be related to special architectures of the Li2FeSiO4/C hollow nanosphere cathode.

Similar content being viewed by others
References
Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295.
Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262.
Goodenough, J. B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176.
Jeong, G.; Kim, Y.-U.; Kim, H.; Kim, Y.-J.; Sohn, H.-J. Prospective materials and applications for Li secondary batteries. Energy Environ. Sci. 2011, 4, 1986–2002.
Nitta, N.; Wu, F. X.; Lee, J. T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264.
Hayner, C. M.; Zhao, X.; Kung, H. H. Materials for rechargeable lithiumion batteries. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 445–471.
Masquelier, C.; Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 2013, 113, 6552–6591.
Ellis, B. L.; Lee, K. T.; Nazar, L. F. Positive electrode materials for Li-ion and Li-batteries. Chem. Mater. 2010, 22, 691–714.
Islam, M. S.; Dominko, R.; Masquelier, C.; Sirisopanaporn, C.; Armstrong, A. R.; Bruce, P. G. Silicate cathodes for lithium batteries: Alternatives to phosphates? J. Mater. Chem. 2011, 21, 9811–9818.
Nishimura, S.; Hayase, S.; Kanno, R.; Yashima, M.; Nakayama, N.; Yamada, A. Structure of Li2FeSiO4. J. Am. Chem. Soc. 2008, 130, 13212–13213.
Boulineau, A.; Sirisopanaporn, C.; Dominko, R.; Armstrong, A. R.; Brucec, P. G.; Masquelier, C. Polymorphism and structural defects in Li2FeSiO4. Dalton Trans. 2010, 39, 6310–6316.
Ni, J. F.; Jiang, Y.; Bi, X. X.; Li, L.; Lu, J. Lithium iron orthosilicate cathode: Progress and perspectives. ACS Energy Lett. 2017, 2, 1771–1781.
Bai, J. Y.; Gong, Z. L.; Lv, D. P.; Li, Y. X.; Zou, H.; Yang, Y. Nanostructured 0.8Li2FeSiO4/0.4Li2SiO3/C composite cathode material with enhanced electrochemical performance for lithium-ion batteries. J. Mater. Chem. 2012, 22, 12128–12132.
Tan, R.; Yang, J. L.; Zheng, J. X.; Wang, K.; Lin, L. P.; Ji, S. P.; Liu, J.; Pan, F. Fast rechargeable all-solid-state lithium ion batteries with high capacity based on nano-sized Li2FeSiO4 cathode by tuning temperature. Nano Energy 2015, 16, 112–121.
Ni, J. F.; Zhang, L.; Fu, S. D.; Savilov, S. V.; Aldoshin, S. M.; Lu, L. A review on integrating nano-carbons into polyanion phosphates and silicates for rechargeable lithium batteries. Carbon 2015, 92, 15–25.
Zhu, Y. Q.; Cao, T.; Li, Z.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Two-dimensional SnO2/graphene heterostructures for highly reversible electrochemical lithium storage. Sci. China Mater., in press, DOI: 10.1007/s40843-018-9324-0.
Rangappa, D.; Murukanahally, K. D.; Tomai, T.; Unemoto, A.; Honma, I. Ultrathin nanosheets of Li2MSiO4 (M = Fe, Mn) as high-capacity Li-ion battery electrode. Nano Lett. 2012, 12, 1146–1151.
Wu, X. Z.; Wang, X. M.; Zhang, Y. X. Nanowormlike Li2FeSiO4-C composites as lithium-ion battery cathodes with superior high-rate capability. ACS Appl. Mater. Interfaces 2013, 5, 2510–2516.
Yang, J. L.; Kang, X. C.; He, D. P.; Zheng, A. M.; Pan, M.; Mu, S. C. Graphene activated 3D-hierarchical flower-like Li2FeSiO4 for high-performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 16567–16573.
Xu, Y. M.; Shen, W.; Zhang, A. L.; Liu, H. M.; Ma, Z. F. Template-free hydrothermal synthesis of Li2FeSiO4 hollow spheres as cathode materials for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 12982–12990.
Zhu, H.; Wu, X. Z.; Zan, L.; Zhang, Y. X. Three-dimensional macroporous graphene Li2FeSiO4 composite as cathode material for lithium-ion batteries with superior electrochemical performances. ACS Appl. Mater. Interfaces 2014, 6, 11724–11733.
Zhang, L.; Ni, J. F.; Wang, W. C.; Guo, J.; Li, L. 3D porous hierarchical Li2FeSiO4/C for rechargeable lithium batteries. J. Mater. Chem. A 2015, 3, 11782–11786.
Qiu, H. L.; Zhu, K.; Li, H. M.; Li, T. T.; Zhang, T.; Yue, H. J.; Wei, Y. J.; Du, F.; Wang, C. Z.; Chen, G. et al. Mesoporous Li2FeSiO4@ordered mesoporous carbon composites cathode material for lithium-ion batteries. Carbon 2015, 87, 365–373.
Li, D. L.; Zhang, W.; Sun, R.; Yong, H.-T.-H.; Chen, G. Q.; Fan, X. Y.; Gou, L.; Mao, Y. Y.; Zhao, K.; Tian, M. Soft-template construction of three-dimensionally ordered inverse opal structure from Li2FeSiO4/C composite nanofibers for high-rate lithium-ion batteries. Nanoscale 2016, 8, 12202–12214.
Ding, Z. P.; Liu, J. T.; Ji, R.; Zeng, X. H.; Yang, S. L.; Pan, A. Q.; Ivey, D. G.; Wei, W. F. Three-dimensionally ordered macroporous Li2FeSiO4/C composite as a high performance cathode for advanced lithium ion batteries. J. Power Sources 2016, 329, 297–304.
Qi, G. G.; Wang, Y. B.; Estevez, L.; Switzer, A. K.; Duan, X. N.; Yang, X. F.; Giannelis, E. P. Facile and scalable synthesis of monodispersed spherical capsules with a mesoporous shell. Chem. Mater. 2010, 22, 2693–2695.
Fan, W.; Zhang, C.; Tjiu, W. W.; Pramoda, K. P.; He, C. B.; Liu, T. X. Graphene-wrapped polyaniline hollow spheres as novel hybrid electrode materials for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 3382–3391.
Wang, X. J.; Feng, J.; Bai, Y. C.; Zhang, Q.; Yin, Y. D. Synthesis, properties, and applications of hollow micro-/nanostructures. Chem. Rev. 2016, 116, 10983–11060.
Lv, D. P.; Bai, J. Y.; Zhang, P.; Wu, S. Q.; Li, Y. X.; Wen, W.; Jiang, Z.; Mi, J. X.; Zhu, Z. Z.; Yang, Y. Understanding the high capacity of Li2FeSiO4: In situ XRD/XANES study combined with first-principles calculations. Chem. Mater. 2013, 25, 2014–2020.
Yang, J. L.; Kang, X. C.; Hu, L.; Gong, X.; Mu, S. C. Nanocrystalline- Li2FeSiO4 synthesized by carbon frameworks as an advanced cathode material for Li-ion batteries. J. Mater. Chem. A 2014, 2, 6870–6878.
Zhang, L. L.; Duan, S.; Yang, X. L.; Liang, G.; Huang, Y. H.; Cao, X. Z.; Yang, J.; Li, M.; Croft, M. C.; Lewis, C. Insight into cobalt-doping in Li2FeSiO4 cathode material for lithium-ion battery. J. Power Sources 2015, 274, 194–202.
Masese, T.; Orikasa, Y.; Tassel, C.; Kim, J.; Minato, T.; Arai, H.; Mori, T.; Yamamoto, K.; Kobayashi, Y.; Kageyama, H. et al. Relationship between phase transition involving cationic exchange and charge-discharge rate in Li2FeSiO4. Chem. Mater. 2014, 26, 1380–1384.
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21503134 and 21406220), the Science Foundation of Ministry of Education of China (No. 413064); PSA Peugeot Citroёn (No.13H100000584); Shanghai Jiao Tong University New Faculty Startup Funds (No.14X10040061); and the Science and Technology Commission of Shanghai Municipality (No.15YF1406500).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shen, S., Zhang, Y., Wei, G. et al. Li2FeSiO4/C hollow nanospheres as cathode materials for lithium-ion batteries. Nano Res. 12, 357–363 (2019). https://doi.org/10.1007/s12274-018-2223-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2223-5