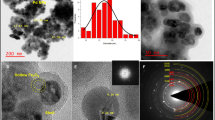
Abstract
Controllable pyrolysis of metal−organic frameworks (MOFs) in confined spaces is a promising strategy for the design and development of advanced functional materials. In this study, Co-Co3O4@carbon composites were synthesized via pyrolysis of a Co-MOFs@glucose polymer (Co-MOFs@GP) followed by partial oxidation of Co nanoparticles (NPs). The pyrolysis of Co-MOFs@GP generated a core–shell structure composed of carbon shells and Co NPs. The controlled partial oxidation of Co NPs formed Co-Co3O4 heterojunctions confined in carbon shells. Compared with Co-MOFs@GP and Co@carbon-n (Co@C-n), Co-Co3O4@carbon-n (Co-Co3O4@C-n) exhibited higher catalytic activity during NaBH4 hydrolysis. Co-Co3O4@C-II provided a maximum specific H2 generation rate of 5,360 mL·min−1·gCo −1 at room temperature due to synergistic interactions between Co and Co3O4 NPs. The Co NPs also endowed Co-Co3O4@C-n with the ferromagnetism needed to complete the magnetic momentum transfer process. This assembly-pyrolysis-oxidation strategy may be an efficient method of preparing novel nanocomposites.

Similar content being viewed by others
References
Wu, R. B.; Wang, D. P.; Rui, X. H.; Liu, B.; Zhou, K.; Law, A. W. K.; Yan, Q. Y.; Wei, J.; Chen, Z. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for highperformance lithium-ion batteries. Adv. Mater. 2015, 27, 3038–3044.
Amali, A. J.; Hoshino, H.; Wu, C.; Ando, M.; Xu, Q. From metal–organic framework to intrinsically fluorescent carbon nanodots. Chem.—Eur. J. 2014, 20, 8279–8282.
Cho, W.; Park, S.; Oh, M. Coordination polymer nanorods of Fe-MIL-88B and their utilization for selective preparation of hematite and magnetite nanorods. Chem. Commun. 2011, 47, 4138–4140.
Aiyappa, H. B.; Pachfule, P.; Banerjee, R.; Kurungot, S. Porous carbons from nonporous MOFs: Influence of ligand characteristics on intrinsic properties of end carbon. Cryst. Growth Des. 2013, 13, 4195–4199.
Tang, J.; Salunkhe, R. R.; Liu, J.; Torad, N. L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal conversion of core–shell metal–organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580.
Srinivas, G.; Krungleviciute, V.; Guo, Z. X.; Yildirim, T. Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 2014, 7, 335–342.
Jia, Y.; Sun, C. H.; Peng, Y.; Fang, W. Q.; Yan, X. C.; Yang, D. J.; Zou, J.; Mao, S. S.; Yao, X. D. Metallic Ni nanocatalyst in situ formed from a metal–organic-framework by mechanochemical reaction for hydrogen storage in magnesium. J. Mater. Chem. A 2015, 3, 8294–8299.
Huang, G.; Zhang, F. F.; Du, X. C.; Qin, Y. L.; Yin, D. M.; Wang, L. M. Metal organic frameworks route to in situ insertion of multiwalled carbon nanotubes in Co3O4 polyhedra as anode materials for lithium-ion batteries. ACS Nano 2015, 9, 1592–1599.
Liu, J. Y.; Yan, J.; Ji, H. Y.; Xu, Y. G.; Huang, L. Y.; Li, Y. P.; Song, Y. H.; Zhang, Q.; Xu, H.; Li, H. M. Controlled synthesis of ordered mesoporous g-C3N4 with a confined space effect on its photocatalytic activity. Mater. Sci. Semicon. Proc. 2016, 46, 59–68.
Manna, P.; Debgupta, J.; Bose, S.; Das, S. K. A mononuclear CoII coordination complex locked in a confined space and acting as an electrochemical water-oxidation catalyst: A “ship-in-a-bottle” approach. Angew. Chem., Int. Ed. 2016, 55, 2425–2430.
Fei, L. F.; Li, X. G.; Bi, W. T.; Zhuo, Z. W.; Wei, W. F.; Sun, L.; Lu, W.; Wu, X. J.; Xie, K. Y.; Wu, C. Z. et al. Graphene/sulfur hybrid nanosheets from a space-confined “Sauna” reaction for high-performance lithium–sulfur batteries. Adv. Mater. 2015, 27, 5936–5942.
Xu, S. K.; Zhang, P.; Li, H. B.; Wei, H. J.; Li, L. M.; Li, B. J.; Wang, X. Y. Ru nanoparticles confined in carbon nanotubes: Supercritical CO2 assisted preparation and improved catalytic performances in hydrogenation of D-glucose. RSC Adv. 2014, 4, 7079–7083.
Zou, F.; Chen, Y. P.; Liu, K. W.; Yu, Z. T.; Liang, W. F.; Bhaway, S. M.; Gao, M.; Zhu, Y. Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano 2016, 10, 377–386.
Chen, Y. H.; Kanan, M. W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts. J. Am. Chem. Soc. 2012, 134, 1986–1989.
Bi, Q. Y.; Lin, J. D.; Liu, Y. M.; He, H. Y.; Huang, F. Q.; Cao, Y. Dehydrogenation of formic acid at room temperature: Boosting palladium nanoparticle efficiency by coupling with pyridinic nitrogen-doped carbon. Angew. Chem., Int. Ed. 2016, 55, 11849–11853.
Xia, W.; Zou, R. Q.; An, L.; Xia, D. G.; Guo, S. J. A metal–organic framework route to in situ encapsulation of Co@Co3O4@C core@bishell nanoparticles into a highly ordered porous carbon matrix for oxygen reduction. Energy Environ. Sci. 2015, 8, 568–576.
Gao, S.; Lin, Y.; Jiao, X. C.; Sun, Y. F.; Luo, Q. Q.; Zhang, W. H.; Li, D. Q.; Yang, J. L.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71.
Wu, G. P.; Wang, J.; Ding, W.; Nie, Y.; Li, L.; Qi, X. Q.; Chen, S. G.; Wei, Z. D. A strategy to promote the electrocatalytic activity of spinels for oxygen reduction by structure reversal. Angew. Chem., Int. Ed. 2016, 55, 1340–1344.
Wang, J.; Zhong, H. X.; Wang, Z. L.; Meng, F. L.; Zhang, X. B. Integrated three-dimensional carbon paper/carbon tubes/cobalt-sulfide sheets as an efficient electrode for overall water splitting. ACS Nano 2016, 10, 2342–2348.
Wang, J.; Zhang, X. B.; Wang, Z. L.; Wang, L. M.; Zhang, Y. Rhodium-nickel nanoparticles grown on graphene as highly efficient catalyst for complete decomposition of hydrous hydrazine at room temperature for chemical hydrogen storage. Energy Environ. Sci. 2012, 5, 6885–6888.
Wang, J.; Qin, Y. L.; Liu, X.; Zhang, X. B. In situ synthesis of magnetically recyclable graphene-supported Pd@Co core–shell nanoparticles as efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Mater. Chem. 2012, 22, 12468–12470.
Qin, Y. L.; Wang, J.; Meng, F. Z.; Wang, L. M.; Zhang, X. B. Efficient PdNi and PdNi@Pd-catalyzed hydrogen generation via formic acid decomposition at room temperature. Chem. Commun. 2013, 49, 10028–10030.
Wang, Z. L.; Hao, X. F.; Jiang, Z.; Sun, X. P.; Xu, D.; Wang, J.; Zhong, H. X.; Meng, F. L.; Zhang, X. B. C and N hybrid coordination derived Co-C-N complex as a highly efficient electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 15070–15073.
Zhou, L. M.; Zhang, T. R.; Tao, Z. L.; Chen, J. Ni nanoparticles supported on carbon as efficient catalysts for the hydrolysis of ammonia borane. Nano Res. 2014, 7, 774–781.
Yan, J. M.; Zhang, X. B.; Akita, T.; Haruta, M.; Xu, Q. One-step seeding growth of magnetically recyclable Au@Co core–shell nanoparticles: Highly efficient catalyst for hydrolytic dehydrogenation of ammonia borane. J. Am. Chem. Soc. 2010, 132, 5326–5327.
Zhu, J.; Li, R.; Niu, W. L.; Wu, Y. J.; Gou, X. L. Facile hydrogen generation using colloidal carbon supported cobalt to catalyze hydrolysis of sodium borohydride. J. Power Sources 2012, 211, 33–39.
Lu, A. L.; Chen, Y. Z.; Jin, J. R.; Yue, G. H.; Peng, D. L. CoO nanocrystals as a highly active catalyst for the generation of hydrogen from hydrolysis of sodium borohydride. J. Power Sources 2012, 220, 391–398.
Niu, W. L.; Ren, D. B.; Han, Y. Y.; Wu, Y. J.; Gou, X. L. Optimizing preparation of carbon supported cobalt catalyst for hydrogen generation from NaBH4 hydrolysis. J. Alloys Compd. 2012, 543, 159–166.
Zhang, H.; Ling, T.; Du, X. W. Gas-phase cation exchange toward porous single-crystal CoO nanorods for catalytic hydrogen production. Chem. Mater. 2015, 27, 352–357.
Mahmood, J.; Jung, S. M.; Kim, S. J.; Park, J.; Yoo, J. W.; Baek, J. B. Cobalt oxide encapsulated in C2N-h 2D network polymer as a catalyst for hydrogen evolution. Chem. Mater. 2015, 27, 4860–4864.
Liu, Y. Y.; Zhang, J.; Zhang, X. J.; Li, B. J.; Wang, X. Y.; Cao, H. Q.; Wei, D.; Zhou, Z. F.; Cheetham, A. K. Magnetic catalysts as nanoactuators to achieve simultaneous momentumtransfer and continuous-flow hydrogen production. J. Mater. Chem. A 2016, 4, 4280–4287.
Xing, C. C.; Liu, Y. Y.; Su, Y. H.; Chen, Y. H.; Hao, S.; Wu, X. L.; Wang, X. Y.; Cao, H. Q.; Li, B. J. Structural evolution of Co-based metal organic frameworks in pyrolysis for synthesis of core–shells on nanosheets: Co@CoOx@carbonrGO composites for enhanced hydrogen generation activity. ACS Appl. Mater. Interfaces 2016, 8, 15430–15438.
Duan, S. S.; Han, G. S.; Su, Y. H.; Zhang, X. Y.; Liu, Y. Y.; Wu, X. L.; Li, B. J. Magnetic Co@g-C3N4 core–shells on rGO sheets for momentum transfer with catalytic activity toward continuous-flow hydrogen generation. Langmuir 2016, 32, 6272–6281.
Yu, G. L.; Sun, J.; Muhammad, F.; Wang, P. Y.; Zhu, G. S. Cobalt-based metal organic framework as precursor to achieve superior catalytic activity for aerobic epoxidation of styrene. RSC Adv. 2014, 4, 38804–38811.
Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O'Keeffe, M.; Yaghi, O. M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943.
Rong, C. B.; Poudyal, N.; Chaubey, G. S.; Nandwana, V.; Liu, Y.; Wu, Y. Q.; Kramer, M. J.; Kozlov, M. E.; Baughman, R. H.; Liu, J. P. High thermal stability of carbon-coated L10-FePt nanoparticles prepared by salt-matrix annealing. J. Appl. Phys. 2008, 103, 07E131.
Qian, J. F.; Sun, F. A.; Qin, L. Z. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223.
Zhou, Y. X.; Chen, Y. Z.; Cao, L. N.; Lu, J. L.; Jiang, H. L. Conversion of a metal–organic framework to N-doped porous carbon incorporating Co and CoO nanoparticles: Direct oxidation of alcohols to esters. Chem. Commun. 2015, 51, 8292–8295.
Lin, K. Y. A.; Hsu, F. K.; Lee, W. D. Magnetic cobaltgraphene nanocomposite derived from self-assembly of MOFs with graphene oxide as an activator for peroxymonosulfate. J. Mater. Chem. A 2015, 3, 9480–9490.
Zhong, S.; Zhan, C. X.; Cao, D. P. Zeolitic imidazolate framework-derived nitrogen-doped porous carbons as high performance supercapacitor electrode materials. Carbon 2015, 8, 51–59.
Liu, Y. W.; Xiao, C.; Lyu, M. J.; Lin, Y.; Cai, W. Z.; Huang, P. C.; Tong, W.; Zou, Y. M.; Xie, Y. Ultrathin Co3S4 nanosheets that synergistically engineer spin states and exposed polyhedra that promote water oxidation under neutral conditions. Angew. Chem., Int. Ed. 2015, 54, 11231–11235.
Gao, S.; Li, G. D.; Liu, Y. P.; Chen, H.; Feng, L. L.; Wang, Y.; Yang, M.; Wang, D. J.; Wang, S.; Zou, X. X. Electrocatalytic H2 production from seawater over Co, N-codoped nanocarbons. Nanoscale 2015, 7, 2306–2316.
Liu, B.; Kong, D. Z.; Zhang, J.; Wang, Y.; Chen, T. P.; Cheng, C. W.; Yang, H. Y. 3D hierarchical Co3O4@Co3S4 nanoarrays as cathode materials for asymmetric pseudocapacitors. J. Mater. Chem. A 2016, 4, 3287–3296.
Yin, P. Q.; Yao, T.; Wu, Y. E.; Zheng, L. R.; Lin, Y.; Liu, W.; Ju, H. X.; Zhu, J. F.; Hong, X.; Deng, Z. X. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805.
Wei, J.; Hu, Y. X.; Liang, Y.; Kong, B.; Zhang, J.; Song, J. C.; Bao, Q. L.; Simon, G. P.; Jiang, S. P.; Wang, H. T. Nitrogen-doped nanoporous carbon/graphene nano-sandwiches: Synthesis and application for efficient oxygen reduction. Adv. Funct. Mater. 2015, 25, 5768–5777.
Huang, Y. C.; Ye, K. H.; Li, H. B.; Fan, W. J.; Zhao, F. Y.; Zhang, Y. M.; Ji, H. B. A highly durable catalyst based on CoxMn3–x O4 nanosheets for low-temperature formaldehyde oxidation. Nano Res. 2016, 9, 3881–3892.
Fu, L.; Liu, Z. M.; Liu, Y. Q.; Han, B. X.; Hu, P. G.; Cao, L. C.; Zhu, D. B. Beaded cobalt oxide nanoparticles along carbon nanotubes: Towards more highly integrated electronic devices. Adv. Mater. 2005, 17, 217–221.
Kong, L. J.; Ren, Z. Y.; Zheng, N. N.; Du, S. C.; Wu, J.; Tang, J. L.; Fu, H. G. Interconnected 1D Co3O4 nanowires on reduced graphene oxide for enzymeless H2O2 detection. Nano Res. 2015, 8, 469–480.
Shi, Q.; Wang, Y. D.; Wang, Z. M.; Lei, Y. P.; Wang, B.; Wu, N.; Han, C.; Xie, S.; Gou, Y. Z. Three-dimensional (3D) interconnected networks fabricated via in-situ growth of N-doped graphene/carbon nanotubes on Co-containing carbon nanofibers for enhanced oxygen reduction. Nano Res. 2016, 9, 317–328.
Ingier-Stocka, E. TG and DTA evaluation of cobalt salts and complexes mixed with activated carbon. J. Therm. Anal. Calorim. 2001, 65, 561–573.
Pu, J.; Li, C. W.; Tang, L.; Li, T. T.; Ling, L.; Zhang, K.; Xu, Y. C.; Li, Q. W.; Yao, Y. G. Impregnation assisted synthesis of 3D nitrogen-doped porous carbon with high capacitance. Carbon 2015, 94, 650–660.
Yang, Y.; Lun, Z. Y.; Xia, G. L.; Zheng, F. C.; He, M. N.; Chen, Q. W. Non-precious alloy encapsulated in nitrogendoped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 2015, 8, 3563–3571.
Zhong, H. X.; Wang, J.; Zhang, Y. W.; Xu, W. L.; Xing, W.; Xu, D.; Zhang, Y. F.; Zhang, X. B. ZIF-8 derived graphenebased nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem., Int. Ed. 2014, 53, 14235–14239.
Liu, Y. Y.; Zhang, W. N.; Li, S. Z.; Cui, C. L.; Wu, J.; Chen, H. Y.; Huo, F. W. Designable yolk–shell nanoparticle@MOF petalous heterostructures. Chem. Mater. 2014, 26, 1119–1125.
Lü, Y. Y.; Zhan, W. W.; He, Y.; Wang, Y. T.; Kong, X. J.; Kuang, Q.; Xie, Z. X.; Zheng, L. S. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195.
Torad, N. L.; Hu, M.; Ishihara, S.; Sukegawa, H.; Belik, A. A.; Imura, M.; Ariga, K.; Sakka, Y.; Yamauchi, Y. Direct synthesis of MOF-derived nanoporous carbon with magnetic Co nanoparticles toward efficient water treatment. Small 2014, 10, 2096–2107.
Lü, Y. Y.; Wang, Y. T.; Li, H. L.; Lin, Y.; Jiang, Z. Y.; Xie, Z. X.; Kuang, Q.; Zheng, L. S. MOF-derived porous Co/C nanocomposites with excellent electromagnetic wave absorption properties. ACS Appl. Mater. Interfaces 2015, 7, 13604–13611.
Metin, Ö.; Mazumder, V.; Özkar, S.; Sun, S. H. Monodisperse nickel nanoparticles and their catalysis in hydrolytic dehydrogenation of ammonia borane. J. Am. Chem. Soc. 2010, 132, 1468–1469.
Seven, F.; Sahiner, N. Metal ion-imprinted hydrogel with magnetic properties and enhanced catalytic performances in hydrolysis of NaBH4 and NH3BH3. Int. J. Hydrogen Energy 2013, 38, 15275–15284.
Ye, W.; Zhang, H. M.; Xu, D. Y.; Ma, L.; Yi, B. L. Hydrogen generation utilizing alkaline sodium borohydride solution and supported cobalt catalyst. J. Power Sources 2007, 164, 544–548.
Liu, B. H.; Li, Z. P.; Suda, S. Nickel- and cobalt-based catalysts for hydrogen generation by hydrolysis of borohydride. J. Alloys Compd. 2006, 415, 288–293.
Feng, K.; Zhong, J.; Zhao, B. H.; Zhang, H.; Xu, L.; Sun, X. H.; Lee, S. T. CuxCo1–x O nanoparticles on graphene oxide as a synergistic catalyst for high-efficiency hydrolysis of ammonia-borane. Angew. Chem., Int. Ed. 2016, 55, 11950–11954.
Akdim, O.; Demirci, U. B.; Miele, P. Deactivation and reactivation of cobalt in hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2011, 36, 13669–13675.
Demirci, U. B.; Miele, P. Cobalt-based catalysts for the hydrolysis of NaBH4 and NH3BH3. Phys. Chem. Chem. Phys. 2014, 16, 6872–6885.
Ozerova, A. M.; Simagina, V. I.; Komova, O. V.; Netskina, O. V.; Odegova, G. V.; Bulavchenko, O. A.; Rudina, N. A. Cobalt borate catalysts for hydrogen production via hydrolysis of sodium borohydride. J. Alloys Compd. 2012, 513, 266–272.
Acknowledgements
Financial supports from the National Natural Science Foundation of China (Nos. 21371154, 21401168, and U1204203) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, Y., Han, G., Zhang, X. et al. Co-Co3O4@carbon core–shells derived from metal−organic framework nanocrystals as efficient hydrogen evolution catalysts. Nano Res. 10, 3035–3048 (2017). https://doi.org/10.1007/s12274-017-1519-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1519-1