Abstract
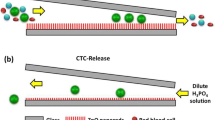
Nanomaterials show promising opportunities to address clinical problems (such as insufficient capture of circulating tumor cells; CTCs) via the high surface area-to-volume ratio and high affinity for biological cells. However, how to apply these nanomaterials as a nano-bio interface in a microfluidic device for efficient CTC capture with high specificity remains a challenge. In the present work, we first found that a titanium dioxide (TiO2) nanorod array that can be conveniently prepared on multiple kinds of substrates has high affinity for tumor cells. Then, the TiO2 nanorod array was vertically grown on the surface of a microchannel with hexagonally patterned Si micropillars via a hydrothermal reaction, forming a new kind of a micro-nano 3D hierarchically structured microfluidic device. The vertically grown TiO2 nanorod array was used as a sensitive nano-bio interface of this 3D hierarchically structured microfluidic device, which showed high efficiency of CTC capture (76.7% ± 7.1%) in an artificial whole-blood sample.

Similar content being viewed by others
References
Gupta, G. P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695.
Weigelt, B.; Peterse, J. L.; Van’t Veer, L. J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602.
Chambers, A. F.; Groom, A. C.; MacDonald, I. C. Metastasis: Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572.
Fidler, I. J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458.
Nakagawa, T.; Martinez, S. R.; Goto, Y.; Koyanagi, K.; Kitago, M.; Shingai, T.; Elashoff, D. A.; Ye, X.; Singer, F. R.; Giuliano, A. E. Detection of circulating tumor cells in earlystage breast cancer metastasis to axillary lymph nodes. Clin. Cancer Res. 2007, 13, 4105–4110.
Cristofanilli, M.; Budd, G. T.; Ellis, M. J.; Stopeck, A.; Matera, J.; Miller, M. C.; Reuben, J. M.; Doyle, G. V.; Allard, W. J.; Terstappen, L. W. M. M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791.
Shaffer, D. R.; Leversha, M. A.; Danila, D. C.; Lin, O.; Gonzalez-Espinoza, R.; Gu, B.; Anand, A.; Smith, K.; Maslak, P.; Doyle, G. V. et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin. Cancer Res. 2007, 13, 2023–2029.
Lu, N. N.; Xie, M.; Wang, J.; Lv, S. W.; Yi, J. S.; Dong, W. G.; Huang, W. H. Biotin-triggered decomposable immunomagnetic beads for capture and release of circulating tumor cells. ACS Appl. Mater. Interfaces 2015, 7, 8817–8826.
Fang, S.; Wang, C.; Xiang, J.; Cheng, L.; Song, X. J.; Xu, L. G.; Peng, R.; Liu, Z. Aptamer-conjugated upconversion nanoprobes assisted by magnetic separation for effective isolation and sensitive detection of circulating tumor cells. Nano Res. 2014, 7, 1327–1336.
He, W.; Wang, H. F.; Hartmann, L. C.; Cheng, J.-X.; Low, P. S. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc. Natl. Acad. Sci. USA 2007, 104, 11760–11765.
Lecharpentier, A.; Vielh, P.; Perez-Moreno, P.; Planchard, D.; Soria, J. C.; Farace, F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer 2011, 105, 1338–1341.
Tan, S. J.; Yobas, L.; Lee, G. Y. H.; Ong, C. N.; Lim, C. T. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed. Microdevices 2009, 11, 883–892.
Adams, A. A.; Okagbare, P. I.; Feng, J.; Hupert, M. L.; Patterson, D.; Göttert, J.; McCarley, R. L.; Nikitopoulos, D.; Murphy, M. C.; Soper, S. A. Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. J. Am. Chem. Soc. 2008, 130, 8633–8641.
Nagrath, S.; Sequist, L. V.; Maheswaran, S.; Bell, D. W.; Irimia, D.; Ulkus, L.; Smith, M. R.; Kwak, E. L.; Digumarthy, S.; Muzikansky, A. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239.
Lin, M.; Chen, J.-F.; Lu, Y.-T.; Zhang, Y.; Song, J. Z.; Hou, S.; Ke, Z. F.; Tseng, H.-R. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc. Chem. Res. 2014, 47, 2941–2950.
Sarioglu, A. F.; Aceto, N.; Kojic, N.; Donaldson, M. C.; Zeinali, M.; Hamza, B.; Engstrom, A.; Zhu, H. L.; Sundaresan, T. K.; Miyamoto, D. T. et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods 2015, 12, 685–691.
Yu, X. L.; Wang, B. R.; Zhang, N. G.; Yin, C. Q.; Chen, H.; Zhang, L. L.; Cai, B.; He, Z. B.; Rao, L.; Liu, W. et al. Capture and release of cancer cells by combining on-chip purification and off-chip enzymatic treatment. ACS App. Mater. Interfaces 2015, 7, 24001–24007.
Whitesides, G. M. The origins and the future of microfluidics. Nature 2006, 442, 368–373.
Stott, S. L.; Hsu, C.-H.; Tsukrov, D. I.; Yu, M.; Miyamoto, D. T.; Waltman, B. A.; Rothenberg, S. M.; Shah, A. M.; Smas, M. E.; Korir, G. K. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397.
Murlidhar, V.; Zeinali, M.; Grabauskiene, S.; Ghannad- Rezaie, M.; Wicha, M. S.; Simeone, D. M.; Ramnath, N.; Reddy, R. M.; Nagrath, S. A radial flow microfluidic device for ultra-high-throughput affinity-based isolation of circulating tumor cells. Small 2014, 10, 4895–4904.
Yoon, H. J.; Kozminsky, M.; Nagrath, S. Emerging role of nanomaterials in circulating tumor cell isolation and analysis. ACS Nano 2014, 8, 1995–2017.
Wang, L. X.; Asghar, W.; Demirci, U.; Wan, Y. Nanostructured substrates for isolation of circulating tumor cells. Nano Today 2013, 8, 374–387.
Li, Y. Q.; Chandran, B. K.; Lim, C. T.; Chen, X. D. Rational design of materials interface for efficient capture of circulating tumor cells. Adv. Sci. 2015, 2, DOI: 10.1002/advs.201500118.
Dvir, T.; Timko, B. P.; Kohane, D. S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22.
Dang, J. M.; Leong, K. W. Myogenic induction of aligned mesenchymal stem cell sheets by culture on thermally responsive electrospun nanofibers. Adv. Mater. 2007, 19, 2775–2779.
Stevens, M. M.; George, J. H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138.
Yang, M. T.; Sniadecki, N. J.; Chen, C. S. Geometric considerations of micro- to nanoscale elastomeric post arrays to study cellular traction forces. Adv. Mater. 2007, 19, 3119–3123.
Wu, C.-H.; Huang, Y.-Y.; Chen, P.; Hoshino, K.; Liu, H. Y.; Frenkel, E. P.; Zhang, J. X.; Sokolov, K. V. Versatile immunomagnetic nanocarrier platform for capturing cancer cells. ACS Nano 2013, 7, 8816–8823.
Li, Y. Y.; Lu, Q. H.; Liu, H. L.; Wang, J. F.; Zhang, P. C.; Liang, H. G.; Jiang, L.; Wang, S. T. Antibody-modified reduced graphene oxide films with extreme sensitivity to circulating tumor cells. Adv. Mater. 2015, 27, 6848–6854.
Yoon, H. J.; Kim, T. H.; Zhang, Z.; Azizi, E.; Pham, T. M.; Paoletti, C.; Lin, J.; Ramnath, N.; Wicha, M. S.; Hayes, D. F. et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat. Nanotechnol. 2013, 8, 735–741.
Li, W.; Wang, J. S.; Ren, J. S.; Qu, X. G. 3D graphene oxide–polymer hydrogel: Near-infrared light-triggered active scaffold for reversible cell capture and on-demand release. Adv. Mater. 2013, 25, 6737–6743.
Yoon, H. J.; Shanker, A.; Wang, Y.; Kozminsky, M.; Jin, Q.; Palanisamy, N.; Burness, M. L.; Azizi, E.; Simeone, D. M.; Wicha, M. S. et al. Tunable thermal-sensitive polymergraphene oxide composite for efficient capture and release of viable circulating tumor cells. Adv. Mater. 2016, 28, 4891–4897.
Yin, S. Y.; Wu, Y. L.; Hu, B. H.; Wang, Y.; Cai, P. Q.; Tan, C. K.; Qi, D. P.; Zheng, L. Y.; Leow, W. R.; Tan, N. S. et al. Three-dimensional graphene composite macroscopic structures for capture of cancer cells. Adv. Mater. Interfaces 2014, 1, DOI: 10.1002/admi.201300043.
Wang, S. T.; Wang, H.; Jiao, J.; Chen, K. J.; Owens, G. E.; Kamei, K. I.; Sun, J.; Sherman, D. J.; Behrenbruch, C. P.; Wu, H. et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew. Chem. 2009, 121, 9132–9135.
Zhang, N. G.; Deng, Y. L.; Tai, Q. D.; Cheng, B. R.; Zhao, L. B.; Shen, Q. L.; He, R. X.; Hong, L. Y.; Liu, W.; Guo, S. S. et al. Electrospun TiO2 nanofiber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Adv. Mater. 2012, 24, 2756–2760.
Liu, X. L.; Chen, L.; Liu, H. L.; Yang, G.; Zhang, P. C.; Han, D.; Wang, S. T.; Jiang, L. Bio-inspired soft polystyrene nanotube substrate for rapid and highly efficient breast cancer-cell capture. NPG Asia Mater. 2013, 5, e63.
Sun, N.; Wang, J.; Ji, L. Y.; Hong, S. N.; Dong, J. J.; Guo, Y. H.; Zhang, K. C.; Pei, R. J. A cellular compatible chitosan nanoparticle surface for isolation and in situ culture of rare number ctcs. Small 2015, 11, 5444–5451.
Sun, N.; Liu, M.; Wang, J.; Wang, Z. L.; Li, X. P.; Jiang, B.; Pei, R. J. Chitosan nanofibers for specific capture and nondestructive release of CTCs assisted by pCBMA brushes. Small 2016, 12, 5090–5097.
Hoshino, K.; Huang, Y.-Y.; Lane, N.; Huebschman, M.; Uhr, J. W.; Frenkel, E. P.; Zhang, X. J. Microchip-based immunomagnetic detection of circulating tumor cells. Lab Chip 2011, 11, 3449–3457.
Meng, J. X.; Zhang, P. C.; Zhang, F. L.; Liu, H. L.; Fan, J. B.; Liu, X. L.; Yang, G.; Jiang, L.; Wang, S. T. A self-cleaning TiO2 nanosisal-like coating toward disposing nanobiochips of cancer detection. ACS Nano 2015, 9, 9284–9291.
He, R. X.; Zhao, L. B.; Liu, Y. M.; Zhang, N. G.; Cheng, B. R.; He, Z. B.; Cai, B.; Li, S. Z.; Liu, W.; Guo, S. S. et al. Biocompatible TiO2 nanoparticle-based cell immunoassay for circulating tumor cells capture and identification from cancer patients. Biomed. Microdevices 2013, 15, 617–626.
Wu, S. L.; Weng, Z. Y.; Liu, X. M.; Yeung, K. W. K.; Chu, P. K. Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5464–5481.
Sun, N.; Li, X.; Wang, Z.; Zhang, R.; Wang, J.; Wang, K.; Pei, R. A multiscale TiO2 nanorod array for ultrasensitive capture of circulating tumor cells. ACS Appl. Mater. Interfaces 2016, 8, 12638–12343.
Qiu, J. C.; Li, J. H.; Wang, S.; Ma, B. J.; Zhang, S.; Guo, W. B.; Zhang, X. D.; Wei, T.; Sang, Y. H.; Liu, H. TiO2 nanorod array constructed nanotopography for regulation of mesenchymal stem cells fate and the realization of location-committed stem cell differentiation. Small 2016, 12, 1770–1778.
Acknowledgements
The authors are thankful for funding from the National Natural Science Foundation of China (Nos. 51402063, 51432005, 61405040, 61505010, 51502018, 31270022, and 81471784), the “100 Talents Program” of the Chinese Academy of Sciences, Beijing City Committee of science and technology (No. Z151100003315010), Beijing Natural Science Foundation (Nos. 2164077 and 2164076), the Fundamental Research Funds of Shandong University (No. 2014QY003), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2015023). The authors also acknowledge the support from the “thousands talents” program for pioneer researchers and his innovation team, and support from the President Funding of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
These authors contributed equally to this work.
Electronic supplementary material
12274_2016_1313_MOESM1_ESM.pdf
A titanium dioxide nanorod array as a high-affinity nano-bio interface of a microfluidic device for efficient capture of circulating tumor cells
Rights and permissions
About this article
Cite this article
Qiu, J., Zhao, K., Li, L. et al. A titanium dioxide nanorod array as a high-affinity nano-bio interface of a microfluidic device for efficient capture of circulating tumor cells. Nano Res. 10, 776–784 (2017). https://doi.org/10.1007/s12274-016-1313-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1313-5