Abstract
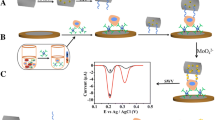
Detection of circulating tumor cells (CTCs) plays an important role in cancer diagnosis and prognosis. In this study, aptamer-conjugated upconversion nanoparticles (UCNPs) are used for the first time as nanoprobes to recognize tumor cells, which are then enriched by attaching with magnetic nanoparticles (MNPs) and placing in the presence of a magnetic field. Owing to the autofluorescencefree nature of upconversion luminescence imaging, as well as the use of magnetic separation to further reduce background signals, our technique allows for highly sensitive detection and collection of small numbers of tumor cells spiked into healthy blood samples, and shows promise for CTC detection in medical diagnostics.

Similar content being viewed by others
References
Ghossein, R. A.; Carusone, L.; Bhattacharya, S. Review: Polymerase chain reaction detection of micrometastases and circulating tumor cells: Application to melanoma, prostate, and thyroid carcinomas. Diagn. Mol. Pathol. 1999, 8, 165–175.
Wittekind, C.; Neid, M. Cancer invasion and metastasis. Oncology 2005, 69 (Suppl. 1), 14–16.
Alunni-Fabbroni, M.; Sandri, M. T. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods 2010, 50, 289–297.
Allard, W. J.; Matera, J.; Miller, M. C.; Repollet, M.; Connelly, M. C.; Rao, C.; Tibbe, A. G. J.; Uhr, J. W.; Terstappen, L. W. M. M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904.
Maltez-da Costa, M.; de la Escosura-Muñiz, A.; Nogués, C.; Barrios, L.; Ibáñez, E.; Merkoci, A. Simple monitoring of cancer cells using nanoparticles. Nano Lett. 2012, 12, 4164–4171.
Galanzha, E. I.; Shashkov, E. V.; Kelly, T.; Kim, J. W.; Yang, L.; Zharov, V. P. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009, 4, 855–860.
Gazouli, M.; Lyberopoulou, A.; Pericleous, P.; Rizos, S.; Aravantinos, G.; Nikiteas, N.; Anagnou, N. P.; Efstathopoulos, E. P. Development of a quantum-dot-labelled magnetic immunoassay method for circulating colorectal cancer cell detection. World J. Gastroenterol. 2012, 18, 4419–4426.
Zieglschmid, V.; Hollmann, C.; Böcher, O. Detection of disseminated tumor cells in peripheral blood. Crit. Rev. Clin. Lab. Sci. 2005, 42, 155–196.
Park, G. S.; Kwon, H.; Kwak, D. W.; Park, S. Y.; Kim, M.; Lee, J. H.; Han, H.; Heo, S.; Li, X. S.; Lee, J. H. et al. Full surface embedding of gold clusters on silicon nanowires for efficient capture and photothermal therapy of circulating tumor cells. Nano Lett. 2012, 12, 1638–1642.
Zhao, L. B.; Lu, Y. T.; Li, F. Q.; Wu, K.; Hou, S.; Yu, J. H.; Shen, Q. L.; Wu, D. X.; Song, M.; Ouyang, W. H. et al. High-purity prostate circulating tumor cell isolation by a polymer nanofiber-embedded microchip for whole exome sequencing. Adv. Mater. 2013, 25, 2897–2902.
Chen, L.; Liu, X. L.; Su, B.; Li, J.; Jiang, L.; Han, D.; Wang, S. T. Aptamer-mediated efficient capture and release of T lymphocytes on nanostructured surfaces. Adv. Mater. 2011, 23, 4376–4380.
Wang, S. T.; Liu, K.; Liu, J.; Yu, Z. T. F.; Xu, X. W.; Zhao, L. B.; Lee, T.; Lee, E. K.; Reiss, J.; Lee, Y. K. et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. Int. Ed. 2011, 50, 3084–3088.
Wu, L.; Wang, J. S.; Ren, J. S.; Qu, X. G. Ultrasensitive telomerase activity detection in circulating tumor cells based on DNA metallization and sharp solid-state electrochemical techniques. Adv. Funct. Mater. 2014, 24, 2727–2733.
Chang, Y. S.; di Tomaso, E.; McDonald, D. M.; Jones, R.; Jain, R. K.; Munn, L. L. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 2000, 97, 14608–14613.
Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–174.
Deng, M. L.; Ma, Y. X.; Huang, S.; Hu, G. F.; Wang, L. Y. Monodisperse upconversion NaYF4 nanocrystals: Syntheses and bioapplications. Nano Res. 2011, 4, 685–694.
Idris, N. M.; Gnanasammandhan, M. K.; Zhang, J.; Ho, P. C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585.
Wang, C.; Cheng, L.; Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials. 2011, 32, 1110–1120.
Cheng, L.; Yang, K.; Zhang, S.; Shao, M. W.; Lee, S.; Liu, Z. Highly-sensitive multiplexed in vivo imaging using pegylated upconversion nanoparticles. Nano Res. 2010, 3, 722–732.
Yang, Y. M.; Shao, Q.; Deng, R. R.; Wang, C.; Teng, X.; Cheng, K.; Cheng, Z.; Huang, L.; Liu, Z.; Liu, X. G. In vitro and in vivo uncaging and bioluminescence imaging by using photocaged upconversion nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 3125–3129.
Zhou, L.; Li, Z. H.; Liu, Z.; Yin, M. L.; Ren, J. S.; Qu, X. G. One-step nucleotide-programmed growth of porous upconversion nanoparticles: Application to cell labeling and drug delivery. Nanoscale. 2014, 6, 1445–1452.
Yang, Y. Upconversion nanophosphors for use in bioimaging, therapy, drug delivery and bioassays. Michromchim. Acta 2014, 181, 263–294.
Chen, F.; Bu, W. B.; Cai, W. B.; Shi, J. L. Engineering upconversion nanoparticles for biomedical imaging and therapy. In Engineering in Translational Medicine. Cai, W., Eds.; Springer: London, 2014; pp. 585–609.
Xiong, L. Q.; Chen, Z. G.; Tian, Q. W.; Cao, T. Y.; Xu, C. J.; Li, F. Y. High contrast upconversion luminescence targeted imaging in vivo using peptide-labeled nanophosphors. Anal. Chem. 2009, 81, 8687–8694.
He, L.; Feng, L. Z.; Cheng, L.; Liu, Y. M.; Li, Z. W.; Peng, R.; Li, Y. G.; Guo, L.; Liu, Z. Multilayer dual-polymer-coated upconversion nanoparticles for multimodal imaging and serum-enhanced gene delivery. ACS Appl. Mater. Interface 2013, 5, 10381–10388.
Zvyagin, A. V.; Song, Z.; Nadort, A.; Sreenivasan, V. K. A.; Deyev, S. M. Luminescent nanomaterials for molecular-specific cellular imaging. In Handbook of Nano-Optics and Nanophotonics. Ohtsu, M., Eds.; Springer: Berlin Heidelberg, 2013; pp. 563–596.
Wang, C.; Cheng, L.; Xu, H.; Liu, Z. Towards whole-body imaging at the single cell level using ultra-sensitive stem cell labeling with oligo-arginine modified upconversion nanoparticles. Biomaterials. 2012, 33, 4872–4881.
Cheng, L.; Wang, C.; Ma, X. X.; Wang, Q. L.; Cheng, Y.; Wang, H.; Li, Y. G.; Liu, Z. Multifunctional upconversion nanoparticles for dual-modal imaging-guided stem cell therapy under remote magnetic control. Adv. Funct. Mater. 2013, 23, 272–280.
Liu, C. H.; Wang, H.; Li, X.; Chen, D. P. Monodisperse, size-tunable and highly efficient β-NaYF4:Yb, Er (Tm) up-conversion luminescent nanospheres: Controllable synthesis and their surface modifications. J. Mater. Chem. 2009, 19, 3546–3553.
Prencipe, G.; Tabakman, S. M.; Welsher, K.; Liu, Z.; Goodwin, A. P.; Zhang, L.; Henry, J.; Dai, H. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J. Am. Chem. Soc. 2009, 131, 4783–4787.
Wang, C.; Ma, X. X.; Ye, S. Q.; Cheng, L.; Yang, K.; Guo, L.; Li, C. H.; Li, Y. G.; Liu, Z. Protamine functionalized single-walled carbon nanotubes for stem cell labeling and in vivo Raman/magnetic resonance/photoacoustic triple-modal imaging. Adv. Funct. Mater. 2012, 22, 2363–2375.
Sun, S. H.; Zeng, H.; Robinson, D. B.; Raoux, S.; Rice, P. M.; Wang, S. X.; Li, G. X. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279.
Lee, H.; Yoon, T. J.; Figueiredo, J. L.; Swirski, F. K.; Weissleder, R. Rapid detection and profiling of cancer cells in fine-needle aspirates. Proc. Natl. Acad. Sci. USA 2009, 106, 12459–12464.
Smith, J. E.; Medley, C. D.; Tang, Z. W.; Shangguan, D.; Lofton, C.; Tan, W. H. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal. Chem. 2007, 79, 3075–3082.
Herr, J. K.; Smith, J. E.; Medley, C. D.; Shangguan, D.; Tan, W. H. Aptamer-conjugated nanoparticles for selective collection and detection of cancer cells. Anal. Chem. 2006, 78, 2918–2924.
Shangguan, D.; Cao, Z. H.; Meng, L.; Mallikaratchy, P.; Sefah, K.; Wang, H.; Li, Y.; Tan, W. H. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J. Proteome Res. 2008, 7, 2133–2139.
Author information
Authors and Affiliations
Corresponding authors
Additional information
These two authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Fang, S., Wang, C., Xiang, J. et al. Aptamer-conjugated upconversion nanoprobes assisted by magnetic separation for effective isolation and sensitive detection of circulating tumor cells. Nano Res. 7, 1327–1336 (2014). https://doi.org/10.1007/s12274-014-0497-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0497-9