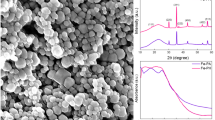
Abstract
The synthesis of semiconductor nanocrystalline networks using weak capping ligands in aqueous media has been demonstrated. Carbohydrates, including ?-cyclodextrin, D-(+)-glucose, D-glucosamine, lactobionic acid, sucrose, and starch were chosen as weak ligands to facilitate the formation of PbTe nanoparticle networks. The nanoparticle size, ranging from 5 nm to 30 nm, can be tuned by manipulating the temperature and concentration. Through a similar strategy, more complicated nanostructures including carbohydrate spheres@PbTe core-shell structures and Te@carbohydrate@PbTe multilayered submicron cables have been fabricated. This is a general approach which can be easily extended to the fabrication of other semiconductor networks, including PbSe and Bi2Te3 using carbohydrates and ethylenediaminetetraacetic acid (EDTA), respectively, as ligands.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937.
Peng, X. G. An essay on synthetic chemistry of colloidal nanocrystals. Nano Res. 2009, 2, 425–447.
Jun, Y. W.; Choi, J. S.; Cheon, J. Shape control of semiconductor and metal oxide nanocrystals through nonhydrolytic colloidal routes. Angew. Chem. Int. Ed. 2006, 45, 3414–3439.
Par, J.; Joo, J.; Kwon, S. G.; Jang, Y.; Hyeon, T. Synthesis of monodisperse spherical nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660.
Burda, C.; Chen, X. B.; Naryanna, R.; El-Sayed, M. A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102.
Korgel, B. A.; Fitzmaurice, D. Self-assembly of silver nanocrystals into two-dimensional nanowire arrays. Adv. Mater. 1998, 10, 661–665.
Pacholski, C.; Kornowski, A.; Weller, H. Self-assembly of ZnO: From nanodots to nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191.
Yu, J. H.; Joo, J.; Park, H. M.; Baik, S.; Kim, Y. W.; Kim, S. C.; Hyeon, T. Synthesis of quantum-sized cubic ZnS nanorods by the oriented attachment mechanism. J. Am. Chem. Soc. 2005, 127, 5662–5670.
Cho, K. S.; Talapin, D. V.; Gaschler, W.; Murray, C. B. Designing PbSe nanowires and nanorings through oriented attachment of nanoparticles. J. Am. Chem. Soc. 2005, 127, 7140–7147.
Tai, G. A.; Guo, W. L.; Zhang, Z. H. Hydrothermal synthesis and thermoelectric transport properties of uniform singlecrystalline pearl-necklace-shaped PbTe nanowires. Cryst. Growth Des. 2008, 8, 2906–2911.
Tang, Z. Y.; Kotov, N. A.; Giersig, M. Spontaneous organization of single CdTe nanoparticles into luminescent nanowires. Science 2002, 297, 237–240.
Kim, F.; Kwan, S.; Akana, J.; Yang, P. Langmuir-Blodgett nanorod assembly. J. Am. Chem. Soc. 2001, 123, 4360–4361.
Xu, X. X.; Wang, X.; Nisar, A.; Liang, X.; Zhuang, J.; Hu, S.; Zhuang, Y. Combinatorial hierarchically ordered 2D architectures self-assembled from nanocrystal building blocks. Adv. Mater. 2008, 20, 3702–3708.
Murray, C. B.; Kagan, C. R.; Bawendi, M. G. Self-organization of CdSe nanocrystallites into 3-dimensional quantum-dot superlattices. Science 1995, 270, 1335–1338.
Nykypanchuk, D.; Maye, M. M.; van der Lelie, D.; Gang, O. DNA-guided crystallization of colloidal nanoparticles. Nature 2008, 451, 549–552.
Park, S. Y.; Lytton-Jean, A. K. R.; Lee, B.; Weigand, S.; Schatz, G. C.; Mirkin, C. A. DNA-programmable nanoparticle crystallization. Nature 2008, 451, 553–556.
Ahniyaz, A.; Sakamoto, Y.; Bergstro, L. Magnetic field-induced assembly of oriented superlattice from maghemite nanocubes. Proc. Nat. Acad. Sci. USA 2007, 104, 17570–17574.
Redl, F. X.; Cho, K. S.; Murray, C. B.; O’Brien, S. Three-dimensional binary superlattices of magnetic nanocrystals and semiconductor quantum dots. Nature 2003, 423, 968–971.
Shevchenko, E. V.; Talapin, D. V.; Murray, C. B.; O’Brien, S. Structural characterization of self-assembled multifunctional binary nanoparticle superlattices. J. Am. Chem. Soc. 2006, 128, 3620–3637.
Zhang, Y. X.; Zeng, H. C. Gold sponges prepared via hydrothermally activated self-assembly of Au nanoparticles. J. Phys. Chem. C 2007, 111, 6970–6975.
Mohanan, J. L.; Arachchige, U. I.; Brock, S. L. Porous semiconductor chalcogenide aerogels. Science 2005, 307, 397–400.
Arachchige, U. I.; Brock, S. L. Sol-gel assembly of CdSe nanoparticles to form porous aerogel networks. J. Am. Chem. Soc. 2006, 128, 7964–7971.
Hanrath, T.; Veldman, D.; Choi, J. J.; Christova, C. G.; Wienk, M. M.; Janssen, R. A. J. PbSe nanocrystal network formation during pyridine ligand displacement. ACS Appl. Mater. Interfaces 2009, 1, 244–250.
Urban, J. J.; Talapin, D. V.; Shevchenko, E. V.; Murray, C. B. Self-assembly of PbTe quantum dots into nanocrystal superlattices and glassy films. J. Am. Chem. Soc. 2006, 128, 3248–3255.
Murphy, J. E.; Beard, M. C.; Norman, A. G.; Ahrenkiel, S. P.; Johnson, J. C.; Yu, P.; Micic, O. I.; Ellingson, R. J.; Nozik, A. J. PbTe colloidal nanocrystals: Synthesis, characterization, and multiple exciton generation. J. Am. Chem. Soc. 2006, 128, 3241–3247.
Dughaish, Z. H. Lead telluride as a thermoelectric material for thermoelectric power generation. Physica B 2002, 322, 205–223.
Sootsman, J. R.; Kong, H.; Uher, C.; D’Angelo, J. J.; Wu, C. I.; Hogan T. P.; Caillat, T.; Kanatzidis, M. G. Large enhancements in the thermoelectric power factor of bulk PbTe at high temperature by synergistic nanostructuring. Angew. Chem. Int. Ed. 2008, 45, 8618–8622.
Zhang, H.; Zhou, Z.; Yang, B. The influence of carboxyl groups on the photoluminescence of mercaptocarboxylic acid-stabilized CdTe nanoparticles. J. Phys. Chem. B 2003, 107, 8–13.
Wang, Q.; Li, H.; Chen, L. Q.; Huang, X. J. Monodispersed hard carbon spherules with uniform nanopores. Carbon 2001, 39, 2211–2214.
Yang, R. Z.; Qiu, X. P.; Zhang, H. R.; Li, J. Q.; Zhu, W. T.; Wang, Z. X.; Huang, X. J.; Chen, L. Q. Monodispersed hard carbon spherules as a catalyst support for the electrooxidation of methanol. Carbon 2005, 43, 11–16.
Sun, X. M.; Li, Y. D. Ga2O3 and GaN semiconductor hollow spheres. Angew. Chem. Int. Ed. 2004, 43, 3827–3831.
Sun, X. M.; Li, Y. D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601.
Qian, H. S.; Yu, S. H.; Luo, L. B.; Gong, J. Y.; Fei, L. F.; Liu, X. M. Synthesis of uniform Te@carbon-rich composite nanocables with photoluminescence properties and carbonaceous nanofibers by the hydrothermal carbonization of glucose. Chem. Mater. 2006, 18, 2102–2108.
Mays, C. W.; Vermaak, J. S.; Kuhlmann-Wilsdorf, D. On surface stress and surface tension. II. Determination of the surface stress of gold. Surf. Sci. 1968, 12, 134–140.
Tong, H.; Zhu, Y. J.; Yang, L. X.; Li, L.; Zhang, L. Lead chalcogenide nanotubes synthesized by biomolecule-assisted self-assembly of nanocrystals at room temperature. Angew. Chem. Int. Ed. 2006, 45, 7739–7742.
Klufers, P.; Schulhmacher, J. Sixteenfold deprotonated ?-cyclodextrin tori as anions in a hexadecanuclear lead(II) alkoxide. Angew. Chem. Int. Ed. 1994, 33, 1863–1865.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is published with open access at Springerlink.com
Electronic supplementary material
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Xiao, Q., Weng, D., Yang, Z. et al. Efficient synthesis of PbTe nanoparticle networks. Nano Res. 3, 685–693 (2010). https://doi.org/10.1007/s12274-010-0030-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-010-0030-8