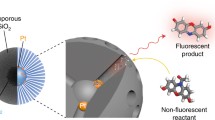
Abstract
Owing to their structural dispersion, the catalytic properties of nanoparticles are challenging to characterize in ensemble-averaged measurements. The single-molecule approach enables studying the catalysis of nanoparticles at the single-particle level with real-time single-turnover resolution. This article reviews our single-molecule fl uorescence studies of single Au-nanoparticle catalysis, focusing on the theoretical formulations for extracting quantitative reaction kinetics from the single-turnover resolution catalysis trajectories. We discuss the single-molecule kinetic formulism of the Langmuir-Hinshelwood mechanism for heterogeneous catalysis, as well as of the two-pathway model for product dissociation reactions. This formulism enables the quantitative evaluation of the heterogeneous reactivity and the differential selectivity of individual nanoparticles that are usually hidden in ensemble measurements. Extension of this formulism to single-molecule catalytic kinetics of oligomeric enzymes is also discussed.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Somorjai, G. A.; Contreras, A. M.; Montano, M.; Rioux, R. M. Clusters, surfaces, and catalysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10577–10583.
Bell, A. T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691.
Burda, C.; Chen, X. B.; Narayanan, R.; El-Sayed, M. A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102.
Heiz, U.; Landman, U. Nanocatalysis; Springer: Berlin, 2007.
Xia, Y. N.; Xiong, Y. J.; Lim, B.; Skrabalak, S. E. Shapecontrolled synthesis of metal nanoparticles: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2008, 48, 60–103.
Tao, A. R.; Habas, S.; Yang, P. D. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325.
Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L. M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791.
Fan, F. -R. F.; Kwak, J.; Bard, A. J. Single molecule electrochemistry. J. Am. Chem. Soc. 1996, 118, 9669–9675.
Fan, F. -R. F.; Bard, A. J. An electrochemical Coulomb staircase: Detection of single electron-transfer events at nanometer electrodes. Science 1997, 277, 1791–1793.
Meier, J.; Friedrich, K. A.; Stimming, U. Novel method for the investigation of single nanoparticle reactivity. Faraday Discuss. 2002, 121, 365–372.
Meier, J.; Schiotz, J.; Liu, P.; Norskov, J. K.; Stimming, U. Nano-scale effects in electrochemistry. Chem. Phys. Lett. 2004, 390, 440–444.
Chen, S. L.; Kucernak, A. Electrocatalysis under conditions of high mass transport: Investigation of hydrogen oxidation on single submicron Pt particles supported on carbon. J. Phys. Chem. B 2004, 108, 13984–13994.
Krapf, D.; Wu, M. -Y.; Smeets, R. M. M.; Zandbergen, H. W.; Dekker, C.; Lemay, S. G. Fabrication and characterization of nanopore-based electrodes with radii down to 2 nm. Nano Lett. 2006, 6, 105–109.
Novo, C.; Funston, A. M.; Mulvaney, P. Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy. Nat. Nanotechnol. 2008, 3, 598–602.
Xu, W.; Kong, J. S.; Yeh, Y. -T. E.; Chen, P. Singlemolecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat. Mater. 2008, 7, 992–996.
Xu, W.; Kong, J. S.; Chen, P. Single-molecule kinetic theory of heterogeneous and enzyme catalysis. J. Phys. Chem. C 2009, 113, 2393–2404.
Xu, W.; Kong, J. S.; Chen, P. Probing the catalytic activity and heterogeneity of Au-nanoparticles at the single-molecule level. Phys. Chem. Chem. Phys. 2009, 11, 2767–2778.
Chen, P.; Xu, W.; Zhou, X. C.; Panda, D.; Kalininskiy, A. Single-nanoparticle catalysis at single-turnover resolution. Chem. Phys. Lett. 2009, 470, 151–157.
Edman, L.; Földes-Papp, Z.; Wennmalm, S.; Rigler, R. The fluctuating enzyme: A single molecule approach. Chem. Phys. 1999, 247, 11–22.
Velonia, K.; Flomenbom, O.; Loos, D.; Masuo, S.; Cotlet, M.; Engelborghs, Y.; Hofkens, J.; Rowan, A. E.; Klafter, J.; Nolte, R. J. M.; de Schryver, F. C. Single-enzyme kinetics of CALB-catalyzed hydrolysis. Angew. Chem. Int. Ed. 2005, 44, 560–564.
English, B. P.; Min, W.; van Oijen, A. M.; Lee, K. T.; Luo, G. B.; Sun, H. Y.; Cherayil, B. J.; Kou, S. C.; Xie, X. S. Everfluctuating single enzyme molecule: Michaelis Menten equation revisited. Nat. Chem. Biol. 2006, 2, 87–94.
Smiley, R. D.; Hammes, G. G. Single molecule studies of enzyme mechanisms. Chem. Rev. 2006, 106, 3080–3094.
Roeffaers, M. B. J.; Sels, B. F.; Uji-i, H.; De Schryver, F. C.; Jacobs, P. A.; De Vos, D. E.; Hofkens, J. Spatially resolved observation of crystal-face-dependent catalysis by single turnover counting. Nature 2006, 439, 572–575.
Sakamoto, M.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Photoreactivity of as-fabricated Au clusters at the single-cluster level. J. Am. Chem. Soc. 2009, 131, 6–7.
Tachikawa, T.; Majima, T. Exploring the spatial distribution and transport behavior of charge carriers in a single titania nanowire. J. Am. Chem. Soc. 2009, 131, 8485–8495.
Satterfield, C. N. Heterogeneous catalysis in practice; McGraw-Hill Book Company: New York, 1980.
Xie, X. S. Single-molecule approach to dispersed kinetics and dynamic disorder: Probing conformational fluctuation and enzymatic dynamics. J. Chem. Phys. 2002, 117, 11024–11032.
Lu, H. P.; Xun, L. Y.; Xie, X. S. Single-molecule enzymatic dynamics. Science 1998, 282, 1877–1882.
Kou, S. C.; Cherayil, B. J.; Min, W.; English, B. P.; Xie, X. S. Single-molecule Michaelis-Menten equations. J. Phys. Chem. B 2005, 109, 19068–19081.
Min, W.; English, B. P.; Luo, G. B.; Cherayil, B. J.; Kou, S. C.; Xie, X. S. Fluctuating enzymes: Lessons from single-molecule studies. Acc. Chem. Res. 2005, 38, 923–931.
Min, W.; Gopich, I. V.; English, B. P.; Kou, S. C.; Xie, X. S.; Szabo, A. When does the Michaelis-Menten equation hold for fluctuating enzymes? J. Phys. Chem. B 2006, 110, 20093–20097.
Xie, S. N. Single-molecule approach to enzymology. Single Mol. 2001, 2, 229–236.
Cao, J. S. Event-averaged measurements of single-molecule kinetics. Chem. Phys. Lett. 2000, 327, 38–44.
Cao, J. S. Single molecule waiting time distribution functions in quantum processes. J. Chem. Phys. 2001, 114, 5137–5140.
Witkoskie, J. B.; Cao, J. S. Single molecule kinetics. I. Theoretical analysis of indicators. J. Chem. Phys. 2004, 121, 6361–6372.
Qian, H.; Elson, E. L. Single-molecule enzymology: Stochastic Michaelis-Menten kinetics. Biophys. Chem. 2002, 101–102, 565–576.
Gopich, I. V.; Szabo, A. Theory of the statistics of kinetic transitions with application to single-molecule enzyme catalysis. J. Chem. Phys. 2006, 124, 154712.
Xue, X.; Liu, F.; Ou-Yang, Z. -C. Single molecule Michaelis-Menten equation beyond quasistatic disorder. Phys. Rev. E 2006, 74, 030902.
Chaudhury, S.; Cherayil, B. J. Dynamic disorder in single-molecule Michaelis-Menten kinetics: The reaction-diffusion formalism in the Wilemski Fixman approximation. J. Chem. Phys. 2007, 127, 105103.
Zhou, Y. J.; Zhuang, X. W. Kinetic analysis of sequential multistep reactions. J. Phys. Chem. B 2007, 111, 13600–13610.
Edman, L.; Rigler, R. Memory landscapes of single-enzyme molecules. Proc. Natl. Acad. Sci. USA 2000, 97, 8266–8271.
Flomenbom, O.; Velonia, K.; Masuo, S.; Loos, D.; Cotlet, M.; Engelborghs, Y.; Hofkens, J.; Rowan, A. E.; Nolte, R. J. M.; van der Auweraer, M.; de Schryver, F. C.; Klafter, J. Stretched exponential decay and correlations in the catalytic activity of fluctuating single lipase molecules. Proc. Natl. Acad. Sci. USA 2005, 102, 2368–2372.
Antikainen, N. M.; Smiley, R. D.; Benkovic, S. J.; Hammes, G. G. Conformation coupled enzyme catalysis: Single-molecule and transient kinetics investigation of dihydrofolate reductase. Biochemistry 2005, 44, 16835–16843.
Gorris, H. H.; Rissin, D. M.; Walt, D. R. Stochastic inhibitor release and binding from single-enzyme molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 17680–17685.
Shi, J.; Dertouzos, J.; Gafni, A.; Steel, D.; Palfey, B. A. Single-molecule kinetics reveals signatures of half-sites reactivity in dihydroorotate dehydrogenase A catalysis. Proc. Natl. Acad. Sci. USA 2006, 103, 5775–5780.
Zhang, Z. Q.; Rajagopalan, P. T. R.; Selzer, T.; Benkovic, S. J.; Hammes, G. G. Single-molecule and transient kinetics investigation of the interaction of dihydrofolate reductase with NADPH and dihydrofolate. Proc. Natl. Acad. Sci. USA 2004, 101, 2764–2769.
Bagshaw, C. R.; Conibear, P. B. Single molecule enzyme kinetics: Application to myosin atpases. Biochem. Soc. Trans. 1999, 27, 33–37.
Paige, M.; Fromm, D. P.; Moerner, W. E. Biomolecular applications of single-molecule measurements: Kinetics and dynamics of a single-enzyme reaction. Proc. Soc. Photo-Opt. Instrum. Eng. 2002, 4634, 92–103.
Fersht, A. Structure and mechanism in protein science: A guide to enzyme catalysis and protein folding; W. H. Freeman and Company: New York, 1998.
van Oijen, A. M.; Blainey, P. C.; Crampton, D. J.; Richardson, C. C.; Ellenberger, T.; Xie, X. S. Single-molecule kinetics of λ exonuclease reveal base dependence and dynamic disorder. Science 2003, 301, 1235–1238.
de Cremer, G.; Roeffaers, M. B. J.; Baruah, M.; Sliwa, M.; Sels, B. F.; Hofkens, J.; De Vos, D. E. Dynamic disorder and stepwise deactivation in a chymotrypsin catalyzed hydrolysis reaction. J. Am. Chem. Soc. 2007, 129, 15458–15459.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Xu, W., Shen, H., Liu, G. et al. Single-molecule kinetics of nanoparticle catalysis. Nano Res. 2, 911–922 (2009). https://doi.org/10.1007/s12274-009-9100-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-009-9100-1