Abstract
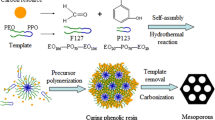
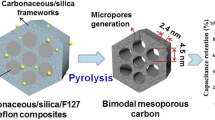
A simple strategy for the synthesis of macro-mesoporous carbonaceous monolith materials has been demonstrated through an organic-organic self-assembly at the interface of an organic scaffold such as polyurethane (PU) foam. Hierarchically porous carbonaceous monoliths with cubic (Im \( \bar 3 \) m) or hexagonal (p6mm) mesostructure were prepared through evaporation induced self-assembly of the mesostructure on the three-dimensional (3-D) interconnecting struts of the PU foam scaffold. The preparation was carried out by using phenol/formaldehyde resol as a carbon precursor, triblock copolymer F127 as a template for the mesostructure and PU foam as a sacrificial monolithic scaffold. Their hierarchical pore system was macroscopically fabricated with cable-like mesostructured carbonaceous struts. The carbonaceous monoliths exhibit macropores of diameter 100–450 μm, adjustable uniform mesopores (3.8–7.5 nm), high surface areas (200–870 m2/g), and large pore volumes (0.17–0.58) cm3/g. Compared with the corresponding evaporation induced self-assembly (EISA) process on a planar substrate, this facile process is a time-saving, labor-saving, space-saving, and highly efficient pathway for mass production of ordered mesoporous materials.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Wan, Y.; Shi, Y. F.; Zhao, D. Y. Supramolecular aggregates as templates: Ordered mesoporous polymers and carbons. Chem. Mater. 2008, 20, 932–945.
Fan, L. Z.; Hu, Y. S.; Maier, J.; Adelhelm, P.; Smarsly, B.; Antonietti, M. High electroactivity of polyaniline in supercapacitors by using a hierarchically porous carbon monolith as a support. Adv. Funct. Mater. 2007, 17, 3083–3087.
Zhao, Y.; Zheng, M. B.; Cao, J. M.; Ke, X. F.; Liu, J. S.; Chen, Y. P.; Tao, J. Easy synthesis of ordered meso/macroporous carbon monolith for use as electrode in electrochemical capacitors. Mater. Lett. 2008, 62, 548–551.
Li, F.; Wang, Z.; Ergang, N. S.; Fyfe, C. A.; Stein, A. Controlling the shape and alignment of mesopores by confinement in colloidal crystals: Designer pathways to silica monoliths with hierarchical porosity. Langmuir 2007, 23, 3996–4004.
Imhof, A.; Pine, D. J. Uniform macroporous ceramics and plastics by emulsion templating. Adv. Mater. 1998, 10, 697–700.
Caruso, F.; Caruso, R. A.; Möhwald, H. Production of hollow microspheres from nanostructured composite particles. Chem. Mater. 1999, 11, 3309–3314.
Yan, H.; Blanford, C. F.; Holland, B. T.; Smyrl, W. H.; Stein, A. General synthesis of periodic macroporous solids by templated salt precipitation and chemical conversion. Chem. Mater. 2000, 12, 1134–1141.
Wakayama, H.; Fukushima, Y. Nanoporous silica prepared with activated carbon molds using supercritical CO2. Chem. Mater. 2000, 12, 756–761.
Deng, Y. H.; Liu, C.; Liu, J.; Zhang, F.; Yu, T.; Zhang, F. Q.; Gu, D., Zhao, D. Y. A novel approach to the construction of 3-D ordered macrostructures with polyhedral particles. J. Mater. Chem. 2008, 18, 408–415.
Taguchi, A.; Smatt, J. H.; Linden, M. Carbon monoliths possessing a hierarchical, fully interconnected porosity. Adv. Mater. 2003, 15, 1209–1211.
Shi, Z. G.; Feng, Y. Q.; Xu, L.; Da, S. L. Zhang, M. Synthesis of a carbon monolith with trimodal pores. Carbon 2003, 41, 2677–2679.
Alvarez, S.; Esquena, J.; Solans, C.; Fuertes, A. B. Meso/macroporous carbon monoliths from polymeric foams. Adv. Eng. Mater. 2004, 6, 897–899.
Lu, A. H.; Li, W. C.; Schmidt, W.; Schuth, F. Fabrication of hierarchically structured carbon monoliths via self-binding and salt templating. Micropor. Mesopor. Mater. 2006, 95, 187–192.
Lu, A. H.; Smatt, J. H.; Backlund, S.; Linden, M. Easy and flexible preparation of nanocasted carbon monoliths exhibiting a multimodal hierarchical porosity. Micropor. Mesopor. Mater. 2004, 72, 59–65.
Wang, L. F.; Lin, S.; Lin, K. F.; Yin, C. Y.; Liang, D. S. Di, Y.; Fan, P. W. Jiang, D. Z.; Xiao, F. S. A facile synthesis of highly ordered mesoporous carbon monolith with mechanically stable mesostructure and superior conductivity from SBA-15 powder. Micropor. Mesopor. Mater. 2005, 85, 136–142.
Deng, Y. H.; Liu, C.; Yu, T.; Liu, F.; Zhang, F. Q.; Wan, Y.; Zhang, L. J.; Wang, C. C.; Tu, B.; Webley, P. A.; Wang, H. T.; Zhao, D. Y. Facile synthesis of hierarchically porous carbons from dual colloidal crystal/block copolymer template approach. Chem. Mater. 2007, 19, 3271–3277.
Feng, P. Y.; Bu, X. H.; Stucky, G. D. Pine, D. J. Monolithic mesoporous silica templated by microemulsion liquid crystals. J. Am. Chem. Soc. 2000, 122, 994–995.
Yang, H. F.; Shi, Q. H.; Tian, B. Z.; Xie, S. H.; Zhang, F. Q. Yan, Y.; Tu, B.; Zhao, D. Y. A fast way for preparing crack-free mesostructured silica monolith. Chem. Mater. 2003, 15, 536–541.
Liang, C. D.; Hong, K. L.; Guiochon, G. A.; Mays, J. W.; Dai, S. Synthesis of a large-scale highly ordered porous carbon film by self-assembly of block copolymers. Angew. Chem. Int. Ed. 2004, 43, 5785–5789.
Liang, C. D.; Dai, S. Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J. Am. Chem. Soc. 2006, 128, 5316 5317.
Meng, Y.; Gu, D.; Zhang, F. Q.; Shi, Y. F.; Yang, H. F.; Li, Z.; Yu, C. Z.; Tu, B.; Zhao, D. Y. Ordered mesoporous polymers and homologous carbon frameworks: Amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 2005, 44, 7053–7059.
Meng, Y.; Gu, D.; Zhang, F. Q.; Shi, Y. F.; Cheng, L.; Feng, D.; Wu, Z. X.; Chen, Z. X.; Wan, Y.; Stein, A.; Zhao, D. Y. A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem. Mater. 2006, 18, 4447–4464.
Huang, Y.; Cai, H. Q.; Yu, T.; Zhang, F. Q.; Zhang, F.; Meng, Y.; Gu, D.; Wan, Y.; Sun, X. L.; Tu, B.; Zhao, D. Y. Formation of mesoporous carbon with a face-centered-cubic Fd \( \bar 3 \) m structure and bimodal architectural pores from the reverse amphiphilic triblock copolymer PPO-PEO-PPO. Angew. Chem. Int. Ed. 2007, 46, 1089–1093.
Deng, Y. H.; Yu, T.; Wan, Y.; Shi, Y. F.; Meng, Y.; Gu, D.; Zhang, L. J.; Huang, Y.; Liu, C.; Wu, X. J.; Zhao, D. Y. Ordered mesoporous silicas and carbons with large accessible pores templated from amphiphilic diblock copolymer poly(ethylene oxide)-b-polystyrene. J. Am. Chem. Soc. 2007, 129, 1690–1697.
Tanka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Synthesis of ordered mesoporous carbons with channel structure from an organic-organic nanocomposite. Chem. Commun. 2005, 16, 2125–2127.
Xue, C. F.; Tu, B.; Zhao, D. Y. Evaporation-induced coating and self-assembly of ordered mesoporous carbon-silica composite monoliths with macroporous architecture on polyurethane foams. Adv. Funct. Mater. 2008, 18, 3914–3921.
Ravikovitch, P. I.; Neimark, A. V. Density functional theory of adsorption in spherical cavities and pore size characterization of templated nanoporous silicas with cubic and three-dimensional hexagonal structures. Langmuir 2002, 18, 1550–1560.
Schmidt, W. Calculation of XRD patterns of simulated FDU-15, CMK-5, and CMK-3 carbon structures. Micropor. Mesopor. Mater. 2009, 117, 372–379.
Inagaki, M.; Morishita, T.; Kuno, A.; Kito, T.; Hirano, M.; Suwa, T.; Kusakawa, K. Carbon foams prepared from polyimide using urethane foam template. Carbon 2004, 42, 497–502.
Trick, K. A.; Saliba, T. E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite. Carbon 1995, 33, 1509 1515.
Konig, A.; Kroke, E. Synthesis of carbon-rich hybrid foam from GAP-modified polyurethane. Propellants Explos. Pyrotech. 2008, 33, 373–380.
Hatchett, D. W.; Kodippili, G.; Kinyanjui, J. M.; Benincasa, F.; Sapochak, L. FTIR analysis of thermally processed PU foam. Polym. Degrad. Stab. 2005, 87, 555–561.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is published with open access at Springerlink.com
Electronic supplementary material
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Xue, C., Tu, B. & Zhao, D. Facile fabrication of hierarchically porous carbonaceous monoliths with ordered mesostructure via an organic organic self-assembly. Nano Res. 2, 242–253 (2009). https://doi.org/10.1007/s12274-009-9022-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-009-9022-y