Abstract
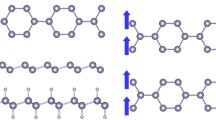
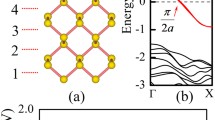
Inspired by recent experimental results, the electronic and magnetic properties of sulfur-passivated ZnO clusters and zigzag nanoribbons have been studied using first principles calculations in the framework of the local spin density approximation. In the case of the ZnO nanoribbons, the sulfur atoms or thiol groups were attached in different ways to the zinc or oxygen atoms located at the edges, whereas in clusters, the sulfur atoms were set on the surface, mainly interacting with atoms with low-coordinate number. After an exhaustive atomic relaxation, we found that a magnetic moment emerges in zigzag nanoribbons both with and without sulfur-passivation on the edges. However, the magnitude of the magnetic moment is very sensitive to sulfur passivation. In particular, we found that when sulfur is attached to the zinc atoms in an alternating fashion along the ribbon edges, the magnetic moment is a maximum (1.4 µB/unit cell). In the case of clusters, we found that the Zn15O15 cluster exhibits a high spin moment of 5.5 µB when capped with sulfur atoms. Our calculations indicate that sulfur-passivating of ZnO nanosystems could be responsible for recently observed ferromagnetic responses.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener model description of ferromagnetism in zinc blende magnetic semiconductors. Science 2000, 287, 1019–1022.
Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C. N. R. Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 2006 74, 161306.
Ronning, C.; Gao, P. X.; Ding, Y.; Wang, Z. L.; Schwen, D. Manganese-doped ZnO nanobelts for spintronics. Appl. Phys. Lett. 2004, 84, 783–785.
Rao, C. N. R.; Deepak, F. L. Absence of ferromagnetism in Mn-and Co-doped ZnO. J. Mater. Chem. 2005, 15, 573–578.
Garcia, M. A.; Merino, J. M.; Fernández Pinel, E.; Quesada, A.; de la Venta, J.; Ruíz González, M. L.; Castro, G. R.; Crespo, P.; Llopis, J.; González Calbet, J. M.; Hernando, A. Magnetic properties of ZnO nanoparticles. Nano Lett. 2007, 7, 1489–1494.
Banerjee, S.; Mandal, M.; Gayathri, N.; Sardar, M. Enhancement of ferromagnetism upon thermal annealing in pure ZnO. Appl. Phys. Lett. 2007, 91, 182501.
Botello-Méndez, A. R.; López Urías, F.; Terrones, M.; Terrones, H. Unpublished results.
Tusche, C.; Meyerheim, H. L.; Kirschner, J. Observation of depolarized ZnO (0001) monolayers: Formation of unreconstructed planar sheets. Phys. Rev. Lett. 2007, 99, 026102.
Botello-Méndez, A. R.; López Urías, F.; Terrones, M.; Terrones, H. Magnetic behavior in zinc oxide zigzag nanoribbons. Nano Lett. 2008, 8, 1562–1565.
Botello-Méndez, A. R.; Martínez-Martínez, M. T.; López Urías, F.; Terrones, M.; Terrones, H. Metallic edges in zinc oxide nanoribbons. Chem. Phys. Lett. 2007, 448, 258–263.
Claeyssens, F.; Freeman, C. L.; Allan, N. L.; Sun, Y.; Ashfold, M. N. R.; Harding, J. H. Growth of ZnO thin films—Experiment and theory. J. Mater. Chem. 2005, 15, 139–148.
Coey, J. M. D. d0 ferromagnetism. Solid State Sci. 2005, 7, 660–667.
Soler, J. M.; Artacho, E.; Gale, J. D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter. 2002, 14, 2745–2779.
Meyer, B.; Marx, D. Density-functional study of the structure and stability of ZnO surfaces. Phys. Rev. B 2003, 67, 035403.
Quantum-ESPRESSO is a community project for high quality quantum-simulation software, based on density functional theory, and coordinated by Paolo Giannozzi. See http://www.quantum-espresso.org and http://www.pwscf.org (Accessed 4 March, 2008).
Janotti, A.; Segev, D.; Van de Walle, C. G. Effects of cation d status on the structural and electronic properties of III-nitride and II-oxide wide-band-gap semiconductors. Phys. Rev. B 2006, 74, 045202.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Botello-Méndez, A.R., López-Urías, F., Terrones, M. et al. Enhanced ferromagnetism in ZnO nanoribbons and clusters passivated with sulfur. Nano Res. 1, 420–426 (2008). https://doi.org/10.1007/s12274-008-8042-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-008-8042-3