Abstract
The gut microbiota that exists in the human gastrointestinal tract is incredibly important for the maintenance of general health as it contributes to multiple aspects of host physiology. Recent research has revealed a dynamic connection between the gut microbiota and the central nervous system, that can influence neurodegenerative diseases (NDs). Indeed, imbalances in the gut microbiota, or dysbiosis, play a vital role in the pathogenesis and progression of human diseases, particularly NDs. Herbal medicine has been used for centuries to treat human diseases, including NDs. These compounds help to relieve symptoms and delay the progression of NDs by improving intestinal barrier function, reducing neuroinflammation, and modulating neurotransmitter production. Notably, herbal medicine can mitigate the progression of NDs by regulating the gut microbiota. Therefore, an in-depth understanding of the potential mechanisms by which herbal medicine regulates the gut microbiota in the treatment of NDs can help explain the pathogenesis of NDs from a novel perspective and propose novel therapeutic strategies for NDs. In this review, we investigate the potential neuroprotective effects of herbal medicine, focusing on its ability to regulate the gut microbiota and restore homeostasis. We also highlight the challenges and future research priorities of the integration of herbal medicine and modern medicine. As the global population ages, access to this information is becoming increasingly important for developing effective treatments for these diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases (NDs) are a group of progressive disorders characterized by the degeneration of neuronal structure and function, resulting in cognitive decline and motor dysfunction (Dugger and Dickson 2017). Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) are the most common NDs that substantially impact the quality of life of patients (Bloem et al. 2021). The pathogenesis of NDs is intricate and involves multiple factors, such as genetic mutations, environmental influences, and aging (Reitz and Mayeux 2014; Hou et al. 2019). Furthermore, the accumulation of misfolded proteins, neuroinflammation, oxidative stress, and mitochondrial dysfunction are common features of NDs (Lin and Beal 2006). As the aging population increases globally, the prevalence of NDs is increasing, and innovative treatments are needed for disease management.
The human gastrointestinal tract is home to a diverse microbial community, collectively known as the gut microbiota. The gut microbiota is a highly complex microbial community that includes bacteria, viruses, fungi, archaea, and microeukaryotes. Although the more proximal regions of the gastrointestinal tract contain diverse microbial communities, the largest biomass is found in the colon (Guarner and Malagelada 2003). Although most of the current research has focused on bacteria, there is growing evidence that these nonbacterial components may also play important roles in health and disease (Sonnenburg and Sonnenburg 2014). The gut microbiota plays an important role in maintaining overall health by regulating immunity, metabolism, and neurological functions (Milani et al. 2017; Cryan et al. 2019). Emerging evidence suggests that alterations in the composition and function of the gut microbiota are influenced by multiple factors, including host genetics and physiology, environmental exposure, age, and dietary factors, which may contribute to the occurrence and development of NDs (Wu et al. 2011, 2016; Sampson and Mazmanian 2015; Sharon et al. 2016). This link between the gut microbiota and brain function, referred to as the gut–brain axis, highlights the essential role of microbial metabolites, immune modulation, and the vagus nerve in facilitating communication between the gut and the central nervous system (CNS) (Agirman et al. 2021; Mayer et al. 2022). In addition, dysbiosis of the gut microbiota has been linked to increased intestinal permeability, systemic inflammation, and susceptibility to neurodegeneration (Sorboni et al. 2022). Moreover, gut microbiota dysbiosis has been found in patients with AD, PD, HD, and ALS, underscoring the importance of maintaining a balanced gut microbial composition for brain health (Gao et al. 2020).
Herbal medicine has a long history of using herbal compounds to treat various diseases (Liao et al. 2023). Recent studies have shown that certain natural components of herbal medicine can modulate the gut microbiota and exert potential therapeutic effects on NDs (Zhang et al. 2021a). These components, such as berberine, baicalein, and ginsenosides, possess antioxidant, anti-inflammatory, and neuroprotective properties (Fan et al. 2020). In addition, these agents can also modulate the gut microbiota by promoting the growth of beneficial bacteria, inhibiting pathogenic bacteria, and regulating the production of microbial metabolites (Krautkramer et al. 2021). These findings suggest the potential of herbal medicine as an alternative or complementary therapy for NDs.
In this review, we consolidate the current evidence regarding the effects of herbal medicine on the gut microbiota in the context of NDs, with a particular focus on AD, PD, and MS. We reveal the mechanisms by which these herbs modulate the gut–brain axis and their potential implications for preventing and treating NDs. Moreover, we also highlight the challenges and directions for future research.
NDs and their pathogenesis
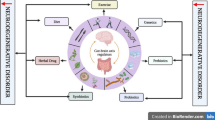
NDs constitute a group of progressive disorders characterized by the loss of neuronal structure and function, resulting in cognitive decline and motor dysfunction. Although the exact pathogenic mechanisms underlying these disorders are incompletely understood, various factors, such as protein misfolding and aggregation, oxidative stress, neuroinflammation, and dysbiosis of the gut microbiota, have been shown to contribute to the development of NDs (Guo et al. 2022) (Fig. 1).
Various factors drive NDs. Under normal homeostatic mechanisms, polypeptides are transcribed and translated from nuclear DNA to extranuclear regions and then properly folded and transported to specific cellular locations. Concurrently, older proteins are labeled and directed to lysosomes for degradation. However, in the event of DNA mutations, translated polypeptides have an increased likelihood of misfolding, resulting in the formation of antidegradation proteins. This leads to the accumulation of misfolded proteins and mitochondrial oxidative stress, driving the pathological progression of NDs. Furthermore, oxidative stress caused by an imbalance between oxidants and antioxidants activates neuronal cells to produce neuroinflammatory factors. These factors synergize with the immune response triggered by pathogen infection, leading to neuroinflammation. Consequently, this inflammation contributes to abnormal biogenesis or protein misfolding, ultimately accelerating the progression of NDs. Additionally, gut microbiota imbalance plays a crucial role in the advancement of NDs
Protein misfolding and aggregation
Neurons depend on precise protein folding to maintain structural and functional integrity. However, protein misfolding can lead to the formation of toxic protein aggregates, which have been implicated in the pathogenesis of NDs (Soto and Pritzkow 2018). For instance, the accumulation of amyloid-beta (Aβ) plaques and tau tangles serve as hallmarks of AD (Panza et al. 2019). Similarly, PD is characterized by the presence of Lewy bodies containing aggregated α-synuclein (Chen et al. 2022b; Karikari et al. 2022). Furthermore, mutant huntingtin protein aggregation is typical of HD, and TAR DNA-binding protein 43 accumulates in ALS (Highet et al. 2020). These toxic aggregates impair the function of neuronal cells and accelerate neuronal cell death. In addition, recent studies have demonstrated that molecular chaperones and the ubiquitin-proteasome system (UPS) play critical roles in maintaining protein homeostasis and preventing protein misfolding and aggregation (Balchin et al. 2016; Eldeeb et al. 2022). Deficiencies in these systems may result in the accumulation of toxic protein aggregates, causing cellular dysfunction and neuronal death.
Oxidative stress
Oxidative stress, which refers to an imbalance between reactive oxygen species (ROS) production and antioxidant defense mechanisms, is widely considered to be a vital factor in the progression of NDs (Teleanu et al. 2022; Tesco and Lomoio 2022). Elevated ROS levels can lead to cellular damage, including lipid, protein, and nucleic acid damage, ultimately leading to neuronal dysfunction and death (Ashleigh et al. 2023). In addition, mitochondrial dysfunction, impaired energy metabolism, and increased ROS production are common features of NDs (Elfawy and Das 2019). Notably, both endogenous and exogenous antioxidants can help neutralize ROS and mitigate oxidative stress, providing a potential therapeutic avenue for these diseases (Kabir et al. 2022). Interestingly, current promising therapeutic strategies for counteracting oxidative stress involve the use of antioxidants and targeting cellular antioxidant mechanisms, such as the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and its downstream targets, to protect against neurodegeneration (Jazvinšćak Jembrek et al. 2021; Ulasov et al. 2022).
Neuroinflammation
Neuroinflammation is a prominent feature of NDs and contributes to neuronal death. For instance, microglia are the primary immune cells in the CNS and play a crucial role in the regulation of neuroinflammation by releasing proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 (Xu et al. 2020; Woodburn et al. 2021). Notably, recent research has revealed the key role of inflammasomes, such as NLRP3, in microglial activation and the development of NDs (Jin et al. 2019; Ahmed et al. 2021). Therefore, targeting neuroinflammation by modulating microglial activation, proinflammatory cytokine production, or inflammasome signaling is a promising therapeutic approach for NDs.
Gut microbiota dysbiosis
Gut microbiota dysbiosis also plays an essential role in the pathogenesis of NDs. There is growing evidence suggesting that alterations in the composition of the gut microbiota can contribute to NDs by affecting neurotransmitter production, and modulating neuroinflammation, immune responses, oxidative stress, protein aggregation, and gut microbiota dysbiosis (Liu et al. 2020b). Furthermore, recent studies have shown the potential benefit of targeting the gut microbiota through dietary interventions, probiotics, and fecal flora transplantation to treat NDs (Peng et al. 2020).
In conclusion, understanding the complex relationships between protein misfolding and aggregation, oxidative stress, neuroinflammation, and gut microbiota dysbiosis in the pathogenesis of NDs is crucial for the development of novel therapeutic approaches targeting these pathways. Recent advances in these areas have shed new light on the development of potential therapeutic targets and strategies for NDs.
Gut microbiota and NDs
The gut–brain axis plays a vital role in maintaining overall health (Fig. 2). This intricate network involves multiple nervous and immune systems and is influenced by the composition of the gut microbiota and its metabolites (Mayer et al. 2022). Notably, the gut–brain axis contributes to regulating various physiological processes, including digestion, metabolism, the immune response, and mood (D’Antongiovanni et al. 2023). Multiple communications of the gut–brain axis occur through several pathways, such as neural connections, hormonal signaling, immune interactions, and microbial metabolites (Ancona et al. 2021). The enteric nervous system, often referred to as the “second brain, ” is a complex network of neurons embedded in the gastrointestinal tract that communicate with the central nervous system (CNS) through the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal axis (HPA) (Furness 2012). Notably, the gut microbiota plays a vital role in this communication by producing neurotransmitters and modulating host metabolism (Strandwitz 2018). Emerging evidence suggests that disturbances in the gut-brain axis contribute to the progression of multiple diseases, including irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and other mental disorders, such as anxiety and depression (Raskov et al. 2016). Therefore, understanding the mechanisms underlying the gut–brain axis and interactions with the gut microbiota has become a vital area of research for ensuring overall health and well-being.
Microbiota-gut-brain axis. The brain and gut are closely linked through neural, metabolic, endocrine, and immune pathways. The brain regulates intestinal health through the vagus nerve, the hypothalamic‒pituitary‒adrenal (HPA) axis, and systemic circulation. At the same time, the intestinal microbiota can regulate the intestinal barrier and nerve cell function through the bacteria itself, microbial-derived metabolites (such as 5-HT and 4EPS), peripheral immune cells, and their secretory factors, thus affecting brain function
The relationship between the gut microbiota and NDs has garnered substantial attention in recent years (Table 1). Accumulating evidence has revealed the vital role of the gut microbiota in the onset and progression of neurological disorders by modulating brain function and behavior (Ma et al. 2019). Alterations in the gut microbiota are associated with several NDs, including AD, PD, HD, and MS. For instance, dysbiosis of the gut microbiota is associated with the production of amyloid‒β plaques and neuroinflammation, both of which are critical factors in the pathogenesis of AD (Shabbir et al. 2021). In addition, certain gut microbial metabolites, such as short-chain fatty acids (SCFAs) and tryptophan derivatives, have shown neuroprotective effects by modulating the immune response of the CNS and enhancing the integrity of the blood-brain barrier (Wang et al. 2023b). In addition, alterations in the gut microbiota have also been reported, with an increase in proinflammatory bacterial species and a decrease in SCFA-producing bacteria in PD patients (Martin-Gallausiaux et al. 2021). The role of the gut microbiota in the pathogenesis of PD is further supported by the observation that α-syn aggregation is a hallmark of PD (Rutsch et al. 2020). Similarly, MS has been linked to alterations in the composition of the gut microbiota, characterized by a decrease in anti-inflammatory and neuroprotective bacterial species. These alterations can affect the blood-brain barrier and promote neuroinflammation, which accelerates the development of MS (Jangi et al. 2016). In conclusion, a deeper understanding of the interactions between the gut microbiota and the gut–brain axis is essential for elucidating the mechanisms of NDs.
Herbal medicine and NDs
Traditional Chinese medicine (TCM) has a history spanning thousands of years and constitutes an essential element of Chinese culture and health care. Central to TCM is the use of herbal medicine, which has played a significant role in preventing and treating various diseases throughout China’s long history (Zhang et al. 2021c). Notably, with its holistic approach, TCM not only focuses on symptoms but also emphasizes balance and harmony among the individual’s body, mind, and environment (Chan et al. 2012). Herbal medicine encompasses thousands of different medicinal plants, minerals, and animal-derived substances. For instance, ancient texts, such as Huangdi Neijing (Yellow Emperor’s Inner Canon) and Shennong Bencaojing (Classic of Herbal Medicine), have laid the foundation for modern TCM, documenting numerous herbs and their therapeutic properties (Zhang et al. 2022b). In recent years, Western scientific research has confirmed the efficacy of herbal medicine, leading to increased global interest in its potential health benefits.
Therapeutic effects of herbal medicine on NDs
The therapeutic principles of herbal medicine are rooted in the ancient TCM concepts of yin and yang, qi (vital energy), and five elements (wood, fire, earth, metal, and water). These principles guide the diagnosis and treatment of diseases by addressing imbalances and deficiencies in the body’s vital energy, blood circulation, and organ functions (Fu et al. 2021; Pun and Chor 2022). Notably, herbal treatment in TCM aims to restore and maintain harmony within the body by addressing the underlying causes of disease rather than just alleviating symptoms. Doctors often use personalized prescriptions that involve the combination of multiple herbs in specific proportions to address the patient’s unique condition and imbalance. Interestingly, this synergistic approach allows for the enhancement of therapeutic effects while minimizing adverse side effects. In TCM, herbs are categorized based on their flavor, temperature, and therapeutic actions, which determine their effects on specific organs and body functions. The therapeutic goals of herbal medicine include strengthening the immune system, promoting blood circulation, eliminating toxins and pathogens, and regulating organ function.
Recently, herbal medicine has gained attention for its potential role in treating NDs. Numerous herbs and formulations have shown promise in clinical trials (Zhu et al. 2022; Liu et al. 2023a) (Table 2). For instance, several herbs have shown potential benefits in alleviating cognitive decline and addressing the pathological hallmarks of AD. These herbs include Huperzia serrata, from which the natural product huperzine A is isolated and it demonstrates acetylcholinesterase inhibition and a reduction in β-amyloid plaques (Friedli and Inestrosa 2021). Ginkgo biloba contains a variety of bioactive components, such as polysaccharides, flavonoids, terpenoid trilactones, and ginkgolic acids, which are known for their antioxidant and anti-inflammatory properties. Polygala tenuifolia contains the main bioactive ingredient senegenin and exhibits neuroprotective effects (Martínez-Solís et al. 2019; Wang et al. 2022a). A commonly used formula for cognitive disorders is Liuwei Dihuang Wan, which aims to nourish yin and strengthen the kidneys, thereby improving memory and cognitive function (Wu et al. 2007). In addition, herbal treatments often focus on replenishing depleted neurotransmitters and protecting dopaminergic neurons in PD patients. Notable herbs, including Uncaria rhynchophylla (Gou Teng with major components, such as rhynchophylline and isorhynchophylline), with its neuroprotective properties (Wang et al. 2018; Yang et al. 2020), and Cistanche deserticola (Rou Cong Rong with total glycosides as the main active components), help improve dopamine levels (Li et al. 2016; Wang et al. 2020). Notably, a frequently prescribed formula for PD is Zhenwu Tang, which warms and tonifies yang while expelling dampness. Herbal medicine focuses on reducing inflammation and enhancing immune function. Scutellaria baicalensis (Huang Qin), which contains the main bioactive ingredients baicalin (BA) and baicalein (BE), is known for its anti-inflammatory and neuroprotective effects, while Astragalus membranaceus (Huang Qi) boosts the immune system in MS (Zhao et al. 2019). Furthermore, a commonly used formula for MS treatment is Bu Zhong Yi Qi Tang, which aims to tonify qi and strengthen the spleen (He et al. 2017). In conclusion, herbal medicine shows promising potential in the prevention and treatment of NDs. More in-depth research and related clinical trials are needed to verify the efficacy and safety of these herbs and formulations and to clarify their potential mechanisms of action.
Mechanisms underlying the effects of herbal medicine in the treatment of NDs
Herbal medicine has been widely used for thousands of years to treat various diseases, especially NDs. It is necessary to reveal the molecular mechanism of TCM treatment of NDs, emphasizing its antioxidant, anti-inflammatory, antiapoptotic, and neuroprotective properties, among others (Fig. 3).
Mechanisms underlying the effects of herbal medicine on NDs. Herbal medicine has the potential to delay the accumulation of amyloid (amyloid β-protein) Aβ protein and Tau protein, regulate the release of central cholinergic and other neurotransmitters, and protect neurons against apoptosis and oxidative stress, thereby providing neuroprotection. Moreover, herbal medicine can also modulate autophagy, enhance mitochondrial function and biogenesis, regulate epigenetic modifications, and influence the gut-brain axis through its interactions with gut microbiota. These multifaceted effects contribute to the prevention and mitigation of NDs
Antioxidative and anti-inflammatory actions
Oxidative stress plays a vital role in the pathogenesis of NDs through protein oxidation, lipid peroxidation, and DNA damage (Esmaeili et al. 2022). Neuroinflammation is closely associated with NDs and exacerbates neuronal dysfunction and death (Scuderi et al. 2020). Herbal medicines can exhibit anti-inflammatory effects through multiple mechanisms, including the inhibition of proinflammatory cytokines, the suppression of microglial activation, and the blockade of inflammatory signaling pathways, thus demonstrating utility in the treatment of NDs (Gong et al. 2020a; Liu et al. 2022b; Li et al. 2023a).
Antiapoptotic and neuroprotective effects
Apoptosis, or programmed cell death, is associated with the pathogenesis of NDs caused by neuronal loss. Herbal medicines can exert antiapoptotic effects by modulating key regulators of apoptosis, including Bcl-2 family proteins, caspases, and p53. For instance, by enhancing the expression of antiapoptotic proteins (e.g., Bcl-2 and Bcl-xL) and reducing the levels of proapoptotic proteins (e.g., Bax and Bad), herbal medicine can restore the balance between cell survival and death, ultimately protecting neurons from degeneration (Liu et al. 2021a).
Herbal medicine provides neuroprotection through a variety of mechanisms, such as promoting neurogenesis, enhancing synaptic plasticity, and regulating the neurotransmitter system (Liu et al. 2021b). For instance, herbal medicine can stimulate neural stem cells to proliferate and differentiate into functional neurons by modulating related signaling pathways such as the Wnt/β-catenin and Notch pathways (Zhou et al. 2014; Li et al. 2023c). Notably, herbal medicine can improve learning and memory functions by facilitating long-term potentiation and modulating neurotransmitter levels, such as glutamate, dopamine, and acetylcholine (Wu et al. 2019; Liu et al. 2020c).
Modulation of protein misfolding and aggregation
Recent research has revealed that herbal medicines can modulate protein misfolding and aggregation, which are hallmarks of several NDs. In addition, certain components of herbal medicine can prevent Aβaggregation, promote Aβ clearance, and inhibit tau hyperphosphorylation in AD (Lu et al. 2021).
Herbal medicine metabolism
In the context of TCM, the gut microbiota acts as an “unseen pharmacist” capable of biotransforming herbal constituents into active metabolites, thereby affecting their bioavailability and pharmacological activity. Ginseng, one of the most widely used herbs in TCM, contains a plethora of saponins known as ginsenosides (Mancuso and Santangelo 2017). While these ginsenosides have numerous reported health benefits, some of their pharmacological actions are attributed to their metabolic products rather than the parent compounds. A key player in this metabolic transformation is compound K, a secondary metabolite formed through the action of intestinal bacteria on primary ginsenosides such as Rb1 and Rb2. Compound K is particularly noteworthy because it has been shown to exert significant anti-inflammatory, antioxidative, and anticancer effects (Choi and Kim 2023; Liu et al. 2023b; Tian et al. 2023). These effects are often more potent than those of the original ginsenosides, highlighting the importance of gut microbial metabolism in enhancing the pharmacological efficacy of ginseng.
Research into the interactions between herbal medicine components and gut microbiota is still evolving. Nevertheless, several studies have underscored the necessity of considering the microbiome when evaluating the pharmacokinetics and pharmacodynamics of herbal medicines. In conclusion, the gut microbiota serves as a critical mediator of the pharmacological effects of herbal medicines, such as ginseng. By transforming inert compounds into bioactive metabolites, these microscopic inhabitants of the human gut significantly influence the therapeutic outcomes of herbal medicine.
Other mechanisms
In addition to the mechanisms discussed above, herbal medicine may also impact other cellular processes relevant to NDs. These processes include the modulation of autophagy, enhancement of mitochondrial function and biogenesis, regulation of epigenetic modifications, and modulation of the gut–brain axis through interaction with the gut microbiota (Zhu et al. 2020a; Wang et al. 2021b).
In conclusion, herbal medicine holds great promise for treating NDs due to its pleiotropic effects on multiple molecular pathways. Further research is warranted to determine the detailed mechanisms underlying the therapeutic effects of herbal medicine and develop effective treatment strategies that can be integrated into mainstream medicine.
The impact of herbal medicine on the gut microbiota
Recent research has revealed the considerable impact of the gut microbiota on multiple aspects of human health, ranging from digestion and metabolism to immunity and mental well-being (Clemente et al. 2012; Fan and Pedersen 2021). Herbal medicine has been shown to influence the composition and function of the gut microbiota. This influence plays a vital role in the therapeutic effects of herbal medicine (Xu et al. 2017) (Fig. 4). Furthermore, recent research has shown that herbal medicine can modulate the composition of the gut microbiota, resulting in increased diversity and favorable shifts in microbial communities. These changes often correlate with improved health outcomes, such as reduced inflammation, enhanced immune responses, and better nutrient absorption (Gong et al. 2020b; Xia et al. 2022a). Moreover, the use of herbal medicine for treating metabolic diseases, such as obesity and type 2 diabetes mellitus, has also been associated with alterations in the composition and function of the gut microbiota (Zhang et al. 2021b). For instance, several mechanisms underlie the effects of herbal medicine on the gut microbiota, including prebiotic effects, antibacterial properties, and immunomodulation.
The effects of herbal medicine on gut microbiota composition. The effects of herbal medicine on gut microbiota were classified as inhabitation, promotion, elimination, and colonization. In addition, the regulatory effects of herbal medicine on gut microbiota composition also include enhancing gut barrier function, inducing host immune response, regulating the composition and metabolism of gut microbiota, changing transport time, etc. More importantly, gut microbiota can enhance our understanding and use of herbal medicine in many ways, which provides more possibilities for herbal medicine to treat diseases
Prebiotic effects
Some Chinese herbs contain nondigestible polysaccharides, oligosaccharides, and other bioactive compounds that serve as substrates for specific bacterial species in the gut. These components act as prebiotics, promoting the growth and activity of beneficial bacteria while inhibiting pathogenic bacteria (Liu et al. 2022c). For instance, Astragalus membranaceus (Huang Qi) and Lycium barbarum (Goji berries) are rich in polysaccharides that specifically stimulate the growth of Bifidobacterium and Lactobacillus (Zhu et al. 2020b; Ding et al. 2021). These prebiotic effects can improve the overall gut microbial composition, enhance SCFA production, and maintain gut barrier integrity, ultimately contributing to improved gut health and disease prevention.
Antibacterial properties
Numerous components of herbal medicine exhibit direct antibacterial effects that can suppress the growth of harmful bacteria while promoting beneficial species. Furthermore, herbal medicine components can also selectively target pathogens without disrupting the overall gut microbial balance. Alkaloids, flavonoids, saponins, and phenolic acids found in various herbal medicine formulations display antibacterial activity against specific pathogenic bacteria, such as Escherichia coli, Staphylococcus aureus, and Helicobacter pylori (Song et al. 2021; Bouchelaghem 2022; Morales-Figueroa et al. 2022). Furthermore, berberine, a bioactive alkaloid found in herbs, such as Coptis chinensis (Huang Lian), inhibits pathogens, such as Escherichia coli and Salmonella typhimurium, while enhancing the abundance of health-promoting bacteria, such as Akkermansia muciniphila (Kong et al. 2012; Dong et al. 2021). Notably, Scutellaria baicalensis (Huang Qin) contains flavonoids with potent antibacterial and anti-inflammatory effects, contributing to a more balanced gut microbiota (Cui et al. 2021). Herbal medicine is beneficial for maintaining a healthy gut microbiota composition and preventing dysbiosis-associated disorders by selectively suppressing the growth of harmful bacteria.
Immunomodulation
The gut microbiota plays an essential role in modulating host immunity, and herbal medicine has been demonstrated to influence the composition and activity of the gut microbiota, subsequently impacting the host immune system. By enhancing the production of antimicrobial peptides and regulating inflammatory cytokines, herbal medicines can maintain the integrity of the gut barrier and prevent pathogen invasion (Liu et al. 2020a; Xia et al. 2022b). In addition, herbal medicine has been demonstrated to enhance the production of SCFAs, the primary metabolic products of gut microbiota fermentation, which have immunomodulatory effects by activating G-protein-coupled receptors and inhibiting histone deacetylases (Feng et al. 2022). Moreover, herbal medicine can also regulate the secretion of cytokines and chemokines, such as IL-10, IL-12, and transforming growth factor-beta (TGF-β), modulating both innate and adaptive immune responses (Fan et al. 2022; Lan et al. 2022; Li et al. 2023b). For instance, ginsenosides, the primary active compounds of Panax ginseng (Ren Shen), have been shown to regulate the expression of Toll-like receptors and other immune-related genes, leading to improved gut barrier function and microbial balance (Liang et al. 2021; Zhou et al. 2021).
One mechanism by which herbal medicine and the gut microbiota modulate immunity is by regulating the balance between different subsets of T cells. For example, certain herbal medicine-derived compounds enhance the proliferation of regulatory T cells (Tregs), which is pivotal for maintaining immunological tolerance and preventing autoimmune diseases. Concurrently, these compounds may also suppress proinflammatory Th17 cells, reducing inflammatory responses that could lead to tissue damage (Ang et al. 2020; Alexander et al. 2022; Ling et al. 2022). Moreover, the gut microbiota can modulate the activity of dendritic cells (DCs), which are essential for antigen presentation and activation of T cells. By influencing DC maturation and cytokine secretion profiles, herbal medicine compounds can shift the immune response toward either a more tolerogenic state or a heightened state of alert against pathogens, depending on the context and requirements of the host (Li et al. 2015; Mirza et al. 2018). Short-chain fatty acids (SCFAs), primarily acetate, propionate, and butyrate, are fermentation byproducts of dietary fibers generated by gut bacteria. SCFAs have been shown to exert multiple beneficial effects on immune homeostasis, including the suppression of inflammatory reactions and enhancement of mucosal barrier function. Many herbs used in TCM are rich in polysaccharides, which serve as prebiotics that promote the growth of SCFA-producing bacteria, thus fostering an anti-inflammatory environment (Tang et al. 2019). Another aspect of the gut microbiota–immune system interaction involves the gut–liver axis. Certain herbal medicines influence the influences gut microbiota composition and metabolic output, which in turn affects the immune-related functions of the liver. This cross-talk is crucial in conditions such as liver cirrhosis and hepatocellular carcinoma, where modulation of immune responses might be therapeutic.
Gut barrier function and bile acid metabolism
Gut barrier function is crucial for maintaining gut homeostasis and bacteria and preventing bacteria and their metabolites from entering systemic circulation. Herbs rich in dietary fibers and polysaccharides promote the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium species. These beneficial microbes can outcompete pathogenic bacteria, enhance gut mucosal immunity, and contribute to the production of short-chain fatty acids (SCFAs), which strengthen the gut barrier (Cheng et al. 2023; Wang et al. 2023a). Herbal medicine can enhance gut barrier function by upregulating the expression of tight junction proteins, such as occludin and claudins, and downregulating the levels of proinflammatory cytokines that disrupt the gut barrier, such as TNF-α and IL-1β (Yu et al. 2020; Liu et al. 2022a). These proteins are critical components of the tight junctions that seal the space between epithelial cells, preventing the leakage of luminal contents into the underlying tissue. Herbal medicine also appears to influence mucin production, which results in the formation of a protective layer on the epithelial surface, further enhancing barrier function. Herbs such as Astragalus membranaceus and Licorice roots have constituents that upregulate mucin gene expression, thereby contributing to the fortification of the mucosal barrier (Qiao et al. 2022). Furthermore, inflammation can compromise tight junction integrity. Herbal medicines possess anti-inflammatory properties and can reduce the levels of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, which are known to disrupt gut barrier function. By downregulating these inflammatory mediators, herbal medicine contributes to maintaining tight junction integrity and preventing barrier dysfunction (Dey 2020). In conditions such as IBD, where the gut barrier is compromised, herbal medicine formulations have been shown to restore microbial balance, reduce inflammation, and improve tight junction protein expression, leading to enhanced barrier integrity and reduced disease severity.
In addition, bile acids are important signaling molecules that influence the composition of the gut microbiota and host metabolism. Herbal medicine has been shown to influence bile acid metabolism, thereby impacting physiological activities. The liver synthesizes primary bile acids, which can be modified by the gut microbiota into secondary bile acids. Herbal medicines such as Chai Hu (Bupleurum chinense) and Yu Jin (Curcuma aromatica) have been shown to regulate enzymes involved in bile acid synthesis, such as cholesterol 7 alpha-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid biosynthesis from cholesterol. By modulating the activity of such enzymes, herbal medicine can alter the composition and pool size of bile acids (Li et al. 2021b, 2022; Cheng et al. 2022). In addition, the gut microbiota plays an essential role in converting primary bile acids into secondary bile acids. Some herbal medicine compounds have antimicrobial properties that can selectively inhibit or promote the growth of specific bacterial species, thus influencing the bile acid transformation process. Other herbal medicine ingredients act as prebiotics, enhancing the growth of beneficial bacteria that contribute to a healthy bile acid profile. (Xiao et al. 2020). Furthermore, bile acids exert their effects through interactions with nuclear receptors, such as the farnesoid X receptor (FXR), and membrane-bound receptors, such as the G protein-coupled bile acid receptor (GPBAR1 or TGR5). Herbal medicines have been found to interact with these receptors, affecting glucose metabolism, lipid homeostasis, and energy balance. For example, certain herbal medicine-derived compounds can activate the FXR, leading to altered expression of genes involved in bile acid homeostasis and energy metabolism. Through the modulation of bile acid metabolism and receptor activation, herbal medicines can potentially affect several physiological processes. These include reducing cholesterol levels, improving glycemic control, and promoting energy expenditure (Hua et al. 2021; Zhang et al. 2022c). Additionally, because bile acids are involved in regulating inflammation and immune responses, herbal medicine-mediated manipulation of bile acid signaling pathways can have implications for treating inflammatory diseases.
Other mechanisms
In addition to the mechanisms mentioned above, additional molecular pathways through which herbal medicine impacts the gut microbiota include the modulation of xenobiotic metabolism, the regulation of gut-derived hormone secretion, and the influence of bacterial metabolites, such as indole, trimethylamine N-oxide (TMAO), and secondary bile acids (Wang et al. 2021a; Zou et al. 2022). These multifaceted effects of herbal medicine on the gut microbiota further contribute to its therapeutic potential for various disorders.
In summary, herbal medicine offers numerous benefits for gut health through its ability to modulate the gut microbiota via diverse molecular mechanisms. However, further research is needed to elucidate the complex interactions among herbal medicine, the gut microbiota, and host physiology, paving the way for new therapeutic applications of herbal medicine in maintaining gut health and preventing disease.
Herbal medicine: potential treatment for NDs through gut microbiota modulation
Recent studies have highlighted the potential of herbal medicine in the treatment of NDs by modulating the gut microbiota and influencing the gut–brain axis (Zhang et al. 2022a). In addition, research has demonstrated that herbal medicine can ameliorate cognitive decline, reduce neuroinflammation, and slow the progression of NDs by regulating the gut microbiota composition. For instance, a recent study revealed that treatment with Ginkgo biloba extract EGb 761 improved cognitive function in AD mice by restoring the balance of the gut microbiota and reducing neuroinflammation (Lautenschlager et al. 2012; Savaskan et al. 2018). Furthermore, another study revealed that berberine, a bioactive alkaloid derived from Coptis chinensis (Huang Lian), alleviated PD symptoms in a mouse model by modulating the gut microbiota and suppressing inflammation (Habtemariam 2020; Qin et al. 2020). Several herbs and formulas have shown promising effects on various NDs by modulating the gut microbiota. The following list highlights some of these therapeutic approaches.
Alzheimer’s disease
Huperzia serrata (huperzine A): This herb has been demonstrated to inhibit acetylcholinesterase activity and reduce β-amyloid plaques in the brain while also improving cognitive function in AD patients. Its therapeutic effect may be associated with its impact on the gut microbiota (Friedli and Inestrosa 2021).
Polygala tenuifolia (senegenin): This herb has exhibited neuroprotective effects and has been reported to modulate the gut microbiota in AD mouse models (Xiong et al. 2022).
Formula: Liuwei Dihuang Wan is a commonly used formula that aims to nourish yin and strengthen the kidneys, thereby improving memory and cognitive function. Recent studies suggest that this formula may also influence the composition of the gut microbiota (Wang et al. 2022c).
Parkinson’s disease
Uncaria rhynchophylla (Gou Teng, alkaloids, terpenoids and flavonoids): This herb possesses neuroprotective properties and has been shown to impact the composition of the gut microbiota in PD rodent models (Lan et al. 2018).
Cistanche deserticola (Rou Cong Rong, phenylethanol glycosides, iridoids, polysaccharides and volatile components): This herb is known to improve dopamine levels and herb has been found to alleviate some PD symptoms by altering the composition of the gut microbiota (Gao et al. 2021).
Formula: Zhenwu Tang is often prescribed for PD patients, aiming to warm and tonify yang while expelling dampness. Its therapeutic effects are potentially associated with gut microbiota regulation (Li 2020).
Multiple sclerosis
Scutellaria baicalensis (Huang Qin, baicalin and baicalein): This herb, known for its anti-inflammatory and neuroprotective effects, has been reported to improve MS symptoms by modulating the gut microbiota (Wang et al. 2022b).
Astragalus membranaceus (Huang Qi, polysaccharides, flavonoids, and saponins): This herb helps boost the immune system and may alleviate MS symptoms by influencing the gut microbiota composition (Peng et al. 2023).
Formula: Bu Zhong Yi Qi Tang is commonly used for MS patients, aiming to tonify qi and strengthen the spleen. Its therapeutic effects have been associated with gut microbiota modulation (Lee et al. 2018).
In summary, herbal medicine holds substantial potential for treating NDs via gut microbiota modulation. However, further research is needed to elucidate the underlying mechanisms involved and identify optimal treatment strategies that harness the full potential of these ancient remedies.
Conclusion and future perspectives
The exploration of herbal medicine about the gut microbiota has opened new vistas in the management of NDs. This review revealed that the gut–brain axis serves as a critical communication pathway through which the gut microbiota can significantly influence brain health and disease progression. The therapeutic potential of herbal medicine for NDs, such as AD, PD, HD, ALS, and MS is becoming increasingly apparent through emerging research. Notably, herbal medicine compounds have been shown to exhibit neuroprotective effects by modulating the gut microbiota composition, reducing systemic inflammation, and enhancing gut barrier integrity. This interplay between herbal medicine and the gut microbiota appears to extend to NDs, where modulation of the gut microbiota could mitigate neuroinflammation and oxidative stress—two central pathological features of NDs. The use of herbal medicine formulations, such as Ginkgo biloba, Ginseng, and Polygala tenuifolia, has demonstrated promising effects in preclinical studies. These herbs contain active metabolites that, once biotransformed by the gut microbiota, can cross the blood–brain barrier and exert therapeutic effects directly within the central nervous system.
Despite these encouraging findings, several challenges persist. Standardization of herbal preparations, understanding of individual phytochemical interactions with diverse gut microbiota constituents, and translation of preclinical results into clinical efficacy remain significant hurdles. Moreover, unraveling the specific components responsible for the beneficial effects is inherently complex due to the multifaceted nature of herbal medicine. The interindividual variability in the gut microbiota also poses a challenge for creating generalized treatments and underscores the need for personalized approaches. Advancing and integrating high-throughput sequencing technologies and metabolomics will enhance our understanding of the gut microbiota in healthy individuals and patients with NDs and elucidate the complex mechanisms underlying the action of herbal medicine on the gut–brain axis. The potential symbiotic relationship between prebiotic and probiotic treatments derived from or inspired by herbal medicine should be explored further to manipulate the gut microbiota beneficially. There is an opportunity to develop novel therapeutic agents targeting the gut–brain axis that can slow or halt the progression of NDs.
In conclusion, the intricate relationships among herbal medicine, the gut microbiota, and NDs hold immense untapped potential for innovative treatment strategies. As we advance our scientific and clinical understanding in this field, the integration of herbal medicine into mainstream ND management offers a hopeful avenue for millions affected by these debilitating conditions.
References
Agirman G, Yu KB, Hsiao EY (2021) Signaling inflammation across the gut-brain axis. Science 374(6571):1087–1092. https://doi.org/10.1126/science.abi6087
Ahmed S, Kwatra M, Ranjan Panda S, Murty USN, Naidu VGM (2021) Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson Disease. Brain Behav Immun 91:142–158. https://doi.org/10.1016/j.bbi.2020.09.017
Alexander M, Ang QY, Nayak RR, Bustion AE, Sandy M, Zhang B, Upadhyay V, Pollard KS, Lynch SV, Turnbaugh PJ (2022) Human gut bacterial metabolism drives Th17 activation and Colitis. Cell Host Microbe 30(1):17–30e9. https://doi.org/10.1016/j.chom.2021.11.001
Ancona A, Petito C, Iavarone I, Petito V, Galasso L, Leonetti A, Turchini L, Belella D, Ferrarrese D, Addolorato G, Armuzzi A, Gasbarrini A, Scaldaferri F (2021) The gut-brain axis in irritable bowel syndrome and inflammatory bowel Disease. Dig Liver Disease 53(3):298–305. https://doi.org/10.1016/j.dld.2020.11.026
Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, Turnbaugh JA, Verdin E, Hall KD, Leibel RL, Ravussin E, Rosenbaum M, Patterson AD, Turnbaugh PJ (2020) Ketogenic diets alter the gut Microbiome resulting in decreased intestinal Th17 cells. Cell 181(6):1263–1275e16. https://doi.org/10.1016/j.cell.2020.04.027
Ashleigh T, Swerdlow RH, Beal MF (2023) The role of mitochondrial dysfunction in Alzheimer’s Disease pathogenesis. Alzheimer’s Dement 19(1):333–342. https://doi.org/10.1002/alz.12683
Auger ML, Meccia J, Phillips AG, Floresco SB (2020) Amelioration of cognitive impairments induced by GABA hypofunction in the male rat prefrontal cortex by direct and indirect dopamine D(1) agonists SKF-81297 and d-govadine. Neuropharmacology 162:107844. https://doi.org/10.1016/j.neuropharm.2019.107844
Balchin D, Hayer-Hartl M, Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353(6294):aac4354. https://doi.org/10.1126/science.aac4354
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397(10291):2284–2303. https://doi.org/10.1016/s0140-6736(21)00218-x
Bouchelaghem S (2022) Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candidaalbicans: a review. Saudi J Biol Sci 29(4):1936–1946. https://doi.org/10.1016/j.sjbs.2021.11.063
Cai Y, Chai Y, Fu Y, Wang Y, Zhang Y, Zhang X, Zhu L, Miao M, Yan T (2021) Salidroside ameliorates Alzheimer’s disease by targeting NLRP3 inflammasome-mediated pyroptosis. Front Aging Neurosci 13:809433. https://doi.org/10.3389/fnagi.2021.809433
Cao Y, Mezzenga R (2019) Food protein amyloid fibrils: origin, structure, formation, characterization, applications and health implications. Adv Colloid Interface Sci 269:334–356. https://doi.org/10.1016/j.cis.2019.05.002
Chai W, Zhang J, Xiang Z, Zhang H, Mei Z, Nie H, Xu R, Zhang P (2022) Potential of nobiletin against Alzheimer’s Disease through inhibiting neuroinflammation. Metab Brain Dis 37(4):1145–1154. https://doi.org/10.1007/s11011-022-00932-7
Chakraborty J, Rajamma U, Jana N, Mohanakumar KP (2015) Quercetin improves the activity of the ubiquitin-proteasomal system in 150Q mutated huntingtin-expressing cells but exerts detrimental effects on neuronal survivability. J Neurosci Res 93(10):1581–1591. https://doi.org/10.1002/jnr.23618
Chan K, Shaw D, Simmonds MS, Leon CJ, Xu Q, Lu A, Sutherland I, Ignatova S, Zhu YP, Verpoorte R, Williamson EM, Duez P (2012) Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J Ethnopharmacol 140(3):469–475. https://doi.org/10.1016/j.jep.2012.01.038
Chen M, Wang T, Yue F, Li X, Wang P, Li Y, Chan P, Yu S (2015) Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience 286:383–392. https://doi.org/10.1016/j.neuroscience.2014.12.003
Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, Weinshenker BG, Rodriguez M, Kantarci OH, Nelson H, Murray JA, Mangalam AK (2016) Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 6:28484. https://doi.org/10.1038/srep28484
Chen M, Li L, Liu C, Song L (2020) Berberine attenuates Aβ-induced neuronal damage through regulating miR-188/NOS1 in Alzheimer’s disease. Mol Cell Biochem 474(1–2):285–294. https://doi.org/10.1007/s11010-020-03852-1
Chen C, Liao J, Xia Y, Liu X, Jones R, Haran J, McCormick B, Sampson TR, Alam A, Ye K (2022a) Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 71(11):2233–2252. https://doi.org/10.1136/gutjnl-2021-326269
Chen R, Gu X, Wang X (2022b) α-Synuclein in Parkinson’s disease and advances in detection. Clinica Chimica Acta Int J Clin Chem 529:76–86. https://doi.org/10.1016/j.cca.2022.02.006
Cheng H, Liu J, Zhang D, Tan Y, Feng W, Peng C (2022) Gut microbiota, bile acids, and nature compounds. Phytother Res 36(8):3102–3119. https://doi.org/10.1002/ptr.7517
Cheng H, Zhang D, Wu J, Liu J, Zhou Y, Tan Y, Feng W, Peng C (2023) Interactions between gut microbiota and polyphenols: a mechanistic and metabolomic review. Phytomedicine 119:154979. https://doi.org/10.1016/j.phymed.2023.154979
Choi S, Kim T (2023) Compound K - an immunomodulator of macrophages in inflammation. Life Sci 323:121700. https://doi.org/10.1016/j.lfs.2023.121700
Christensen LFB, Jensen KF, Nielsen J, Vad BS, Christiansen G, Otzen DE (2019) Reducing the amyloidogenicity of functional amyloid protein FapC increases its ability to inhibit α-synuclein fibrillation. ACS Omega 4(2):4029–4039. https://doi.org/10.1021/acsomega.8b03590
Ciminelli BM, Menduti G, Benussi L, Ghidoni R, Binetti G, Squitti R, Rongioletti M, Nica S, Novelletto A, Rossi L, Malaspina P (2020) Polymorphic genetic markers of the GABA catabolism pathway in Alzheimer’s Disease. J Alzheimers Dis 77(1):301–311. https://doi.org/10.3233/jad-200429
Clemente JC, Ursell LK, Parfrey LW, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270. https://doi.org/10.1016/j.cell.2012.01.035
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG (2019) The Microbiota-Gut-Brain Axis. Physiol Rev 99(4):1877–2013. https://doi.org/10.1152/physrev.00018.2018
Cui L, Guan X, Ding W, Luo Y, Wang W, Bu W, Song J, Tan X, Sun E, Ning Q, Liu G, Jia X, Feng L (2021) Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative Colitis by improving intestinal barrier function and modulating gut microbiota. Int J Biol Macromol 166:1035–1045. https://doi.org/10.1016/j.ijbiomac.2020.10.259
D’Antongiovanni V, Pellegrini C, Antonioli L, Ippolito C, Segnani C, Benvenuti L, D’Amati A, Errede M, Virgintino D, Fornai M, Bernardini N (2023) Enteric glia and brain astroglia: Complex Communication in Health and Disease along the Gut-Brain Axis. The neuroscientist: a review journal bringing neurobiology. Neurol Psychiatry:10738584231163460. https://doi.org/10.1177/10738584231163460
Dey P (2020) Targeting gut barrier dysfunction with phytotherapies: effective strategy against chronic Diseases. Pharmacol Res 161:105135. https://doi.org/10.1016/j.phrs.2020.105135
Ding G, Gong Q, Ma J, Liu X, Wang Y, Cheng X (2021) Immunosuppressive activity is attenuated by Astragalus polysaccharides through remodeling the gut microenvironment in Melanoma mice. Cancer Sci 112(10):4050–4063. https://doi.org/10.1111/cas.15078
Dong C, Yu J, Yang Y, Zhang F, Su W, Fan Q, Wu C, Wu S (2021) Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed Pharmacother 139:111595. https://doi.org/10.1016/j.biopha.2021.111595
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative Diseases. Cold Spring Harb Perspect Biol 9(7). doi: 10.1101/cshperspect.a028035.
Eldeeb MA, Thomas RA, Ragheb MA, Fallahi A, Fon EA (2022) Mitochondrial quality control in health and in Parkinson’s Disease. Physiol Rev 102(4):1721–1755. https://doi.org/10.1152/physrev.00041.2021
Elfawy HA, Das B (2019) Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative Disease: etiologies and therapeutic strategies. Life Sci 218:165–184. https://doi.org/10.1016/j.lfs.2018.12.029
Esmaeili Y, Yarjanli Z, Pakniya F, Bidram E, Łos MJ, Eshraghi M, Klionsky DJ, Ghavami S, Zarrabi A (2022) Targeting autophagy, oxidative stress, and ER stress for neurodegenerative Disease treatment. J Controlled Release 345:147–175. https://doi.org/10.1016/j.jconrel.2022.03.001
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and Disease. Nat Rev Microbiol 19(1):55–71. https://doi.org/10.1038/s41579-020-0433-9
Fan W, Huang Y, Zheng H, Li S, Li Z, Yuan L, Cheng X, He C, Sun J (2020) Ginsenosides for the treatment of metabolic syndrome and Cardiovascular Diseases: Pharmacology and mechanisms. Biomed Pharmacother 132:110915. https://doi.org/10.1016/j.biopha.2020.110915
Fan M, Gu X, Zhang W, Shen Q, Zhang R, Fang Q, Wang Y, Guo X, Zhang X, Liu X (2022) Atractylenolide I ameliorates cancer Cachexia through inhibiting biogenesis of IL-6 and tumour-derived extracellular vesicles. J cachexia Sarcopenia Muscle 13(6):2724–2739. https://doi.org/10.1002/jcsm.13079
Feng W, Zhu L, Shen H (2022) Traditional Chinese Medicine alleviates Ulcerative Colitis via modulating gut microbiota. Evidence-Based Complement Altern 2022:8075344. https://doi.org/10.1155/2022/8075344
Friedli MJ, Inestrosa NC (2021) Huperzine A and Its Neuroprotective Molecular Signaling in Alzheimer’s Disease. Molecules 26(21). doi: 10.3390/molecules26216531.
Fu R, Li J, Yu H, Zhang Y, Xu Z, Martin C (2021) The Yin and Yang of traditional Chinese and western medicine. Med Res Rev 41(6):3182–3200. https://doi.org/10.1002/med.21793
Fujita K, Seike T, Yutsudo N, Ohno M, Yamada H, Yamaguchi H, Sakumi K, Yamakawa Y, Kido MA, Takaki A, Katafuchi T, Tanaka Y, Nakabeppu Y, Noda M (2009) Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s Disease. PLoS ONE 4(9):e7247. https://doi.org/10.1371/journal.pone.0007247
Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Reviews Gastroenterol Hepatol 9(5):286–294. https://doi.org/10.1038/nrgastro.2012.32
Gao K, Mu CL, Farzi A, Zhu WY (2020) Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr 11(3):709–723. https://doi.org/10.1093/advances/nmz127
Gao Y, Li B, Liu H, Tian Y, Gu C, Du X, Bu R, Gao J, Liu Y, Li G (2021) Cistanche deserticola polysaccharides alleviate cognitive decline in aging model mice by restoring the gut microbiota-brain axis. Aging 13(11):15320–15335. https://doi.org/10.18632/aging.203090
Gong J, Sun D (2022) Study on the mechanism of curcumin to reduce the inflammatory response of temporal lobe in Alzheimer’s Disease by regulating miR-146a. Minerva Med 113(1):109–118. https://doi.org/10.23736/s0026-4806.20.06463-0
Gong G, Guan YY, Zhang ZL, Rahman K, Wang SJ, Zhou S, Luan X, Zhang H (2020a) Isorhamnetin: a review of pharmacological effects. Biomed Pharmacother 128:110301. https://doi.org/10.1016/j.biopha.2020.110301
Gong X, Li X, Bo A, Shi RY, Li QY, Lei LJ, Zhang L, Li MH (2020b) The interactions between gut microbiota and bioactive ingredients of traditional Chinese medicines: a review. Pharmacol Res 157:104824. https://doi.org/10.1016/j.phrs.2020.104824
Guarner F, Malagelada JR (2003) Gut flora in health and Disease. Lancet 361(9356):512–519. https://doi.org/10.1016/s0140-6736(03)12489-0
Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, Li J (2022) Aging and aging-related Diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Therapy 7(1):391. https://doi.org/10.1038/s41392-022-01251-0
Habtemariam S (2020) Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res 155:104722. https://doi.org/10.1016/j.phrs.2020.104722
He M, Chen W, Wang M, Wu Y, Zeng J, Zhang Z, Shen S, Jiang J (2017) Simultaneous determination of multiple bioactive components of Bu-zhong-Yi-qi-Tang in rat tissues by LC-MS/MS: application to a tissue distribution study. J Chromatogr B 1044–1045:177–184. https://doi.org/10.1016/j.jchromb.2017.01.023
Highet B, Dieriks BV, Murray HC, Faull RLM, Curtis MA (2020) Huntingtin Aggregates in the olfactory bulb in Huntington’s Disease. Front Aging Neurosci 12:261. https://doi.org/10.3389/fnagi.2020.00261
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA (2019) Ageing as a risk factor for neurodegenerative Disease. Nat Reviews Neurol 15(10):565–581. https://doi.org/10.1038/s41582-019-0244-7
Hua YL, Jia YQ, Zhang XS, Yuan ZW, Ji P, Hu JJ, Wei YM (2021) Baitouweng Tang ameliorates DSS-induced ulcerative colitis through the regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. Biomedicine and Pharmacotherapy 137:111320 doi: 10.1016/j.biopha.2021.111320.
Ip P, Sharda PR, Cunningham A, Chakrabartty S, Pande V, Chakrabartty A (2017) Quercitrin and quercetin 3-β-d-glucoside as chemical chaperones for the A4V SOD1 ALS-causing mutant. Protein Eng Des Sel 30(6):431–440. https://doi.org/10.1093/protein/gzx025
Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topçuolu BD, Holden J, Kivisäkk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7:12015. https://doi.org/10.1038/ncomms12015
Jazvinšćak Jembrek M, Oršolić N, Mandić L, Sadžak A, Šegota S (2021) Anti-Oxidative, anti-inflammatory and anti-apoptotic effects of flavonols: Targeting Nrf2, NF-κB and p53 pathways in Neurodegeneration. Antioxidants 10(10). doi: 10.3390/antiox10101628.
Jin X, Liu MY, Zhang DF, Zhong X, Du K, Qian P, Yao WF, Gao H, Wei MJ (2019) Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci Ther 25(5):575–590. https://doi.org/10.1111/cns.13086
Kabir MT, Rahman MH, Shah M, Jamiruddin MR, Basak D, Al-Harrasi A, Bhatia S, Ashraf GM, Najda A, El-Kott AF, Mohamed HRH, Al-Malky HS, Germoush MO, Altyar AE, Alwafai EB, Ghaboura N, Abdel-Daim MM (2022) Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed Pharmacother 146:112610. https://doi.org/10.1016/j.biopha.2021.112610
Karikari AA, McFleder RL, Ribechini E, Blum R, Bruttel V, Knorr S, Gehmeyr M, Volkmann J, Brotchie JM, Ahsan F, Haack B, Monoranu CM, Keber U, Yeghiazaryan R, Pagenstecher A, Heckel T, Bischler T, Wischhusen J, Koprich JB, Lutz MB, Ip CW (2022) Neurodegeneration by α-synuclein-specific T cells in AAV-A53T-α-synuclein Parkinson’s disease mice. Brain Behav Immun 101:194–210. https://doi.org/10.1016/j.bbi.2022.01.007
Kong WJ, Xing XY, Xiao XH, Zhao YL, Wei JH, Wang JB, Yang RC, Yang MH (2012) Effect of berberine on Escherichiacoli, Bacillussubtilis, and their mixtures as determined by isothermal microcalorimetry. Appl Microbiol Biotechnol 96(2):503–510. https://doi.org/10.1007/s00253-012-4302-y
Krautkramer KA, Fan J, Bäckhed F (2021) Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol 19(2):77–94. https://doi.org/10.1038/s41579-020-0438-4
Lam PY, Ko KM (2012) Beneficial effect of (-)schisandrin B against 3-nitropropionic acid-induced cell death in PC12 cells. BioFactors 38(3):219–225. https://doi.org/10.1002/biof.1009
Lan YL, Zhou JJ, Liu J, Huo XK, Wang YL, Liang JH, Zhao JC, Sun CP, Yu ZL, Fang LL, Tian XG, Feng L, Ning J, Zhang BJ, Wang C, Zhao XY, Ma XC (2018) Uncaria rhynchophylla ameliorates Parkinson’s disease by inhibiting HSP90 expression: insights from quantitative proteomics. Cell Physiol Biochem 47(4):1453–1464. https://doi.org/10.1159/000490837
Lan T, Jiang S, Zhang J, Weng Q, Yu Y, Li H, Tian S, Ding X, Hu S, Yang Y, Wang W, Wang L, Luo D, Xiao X, Piao S, Zhu Q, Rong X, Guo J (2022) Breviscapine alleviates NASH by inhibiting TGF-β-activated kinase 1-dependent signaling. Hepatology 76(1):155–171. https://doi.org/10.1002/hep.32221
Lautenschlager NT, Ihl R, Müller WE (2012) Ginkgo biloba extract EGb 761® in the context of current developments in the diagnosis and treatment of age-related cognitive decline and Alzheimer’s disease: a research perspective. Int Psychogeriatr 24(Suppl 1):S46-50. https://doi.org/10.1017/s1041610212001019
Lee LW, Lin HJ, Huang ST (2018) Management of IFN-beta-induced flu-like symptoms with Chinese herbal medicine in a patient with multiple sclerosis: a case report. Complement Ther Med 36:123–128. https://doi.org/10.1016/j.ctim.2017.12.011
Li H (2020) Advances in anti hepatic fibrotic therapy with traditional Chinese medicine herbal formula. J Ethnopharmacol 251:112442. https://doi.org/10.1016/j.jep.2019.112442
Li FQ, Wang T, Pei Z, Liu B, Hong JS (2005) Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. J Neural Transm 112(3):331–347. https://doi.org/10.1007/s00702-004-0213-0
Li YY, Cui JG, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ (2011) Increased expression of miRNA-146a in Alzheimer’s disease transgenic mouse models. Neurosci Lett 487(1):94–98. https://doi.org/10.1016/j.neulet.2010.09.079
Li J, Li J, Zhang F (2015) The immunoregulatory effects of Chinese herbal medicine on the maturation and function of dendritic cells. J Ethnopharmacol 171:184–195. https://doi.org/10.1016/j.jep.2015.05.050
Li Z, Lin H, Gu L, Gao J, Tzeng CM (2016) Herba cistanche (Rou Cong-Rong): one of the best pharmaceutical gifts of traditional Chinese medicine. Front Pharmacol 7:41. https://doi.org/10.3389/fphar.2016.00041
Li X, Smid SD, Lin J, Gong Z, Chen S, You F, Zhang Y, Hao Z, Lin H, Yu X, Jin X (2019) Neuroprotective and anti-amyloid β effect and main chemical profiles of white tea: comparison against green, oolong and black tea. Molecules 24(10):1926. https://doi.org/10.3390/molecules24101926
Li X, Su Y, Li N, Zhang FR, Zhang N (2021a) Berberine attenuates MPP(+)-induced neuronal injury by regulating LINC00943/miR-142-5p/KPNA4/NF-κB pathway in SK-N-SH cells. Neurochem Res 46(12):3286–3300. https://doi.org/10.1007/s11064-021-03431-w
Li Y, Ji X, Wu H, Li X, Zhang H, Tang D (2021b) Mechanisms of traditional Chinese medicine in modulating gut microbiota metabolites-mediated lipid metabolism. J Ethnopharmacol 278:114207. https://doi.org/10.1016/j.jep.2021.114207
Li X, Zhao W, Xiao M, Yu L, Chen Q, Hu X, Zhao Y, Xiong L, Chen X, Wang X, Ba Y, Guo Q, Wu X (2022) Penthorum chinense pursh. Extract attenuates non-alcholic fatty liver disease by regulating gut microbiota and bile acid metabolism in mice. J Ethnopharmacol 294:115333. https://doi.org/10.1016/j.jep.2022.115333
Li L, Peng P, Ding N, Jia W, Huang C, Tang Y (2023a) Oxidative stress, inflammation, gut dysbiosis: what can polyphenols do in inflammatory bowel. Disease? Antioxid. https://doi.org/10.3390/antiox12040967
Li L, Tian H, Zhang Z, Ding N, He K, Lu S, Liu R, Wu P, Wang Y, He B, Luo M, Peng P, Yang M, Nice EC, Huang C, Xie N, Wang D, Gao W (2023b) Carrier-free nanoplatform via evoking pyroptosis and Immune response against breast cancer. ACS Appl Mater Interfaces 15(1):452–468. https://doi.org/10.1021/acsami.2c17579
Li S, Li Y, Sun W, Qin Z, Lu Y, Song Y, Ga M, Yuan F, Liu Q (2023c) Sanwei DouKou decoction ameliorate Alzheimer Disease by increasing endogenous neural stem cells proliferation through the Wnt/β-catenin signalling pathway. J Ethnopharmacol 309:116364. https://doi.org/10.1016/j.jep.2023.116364
Liang W, Zhou K, Jian P, Chang Z, Zhang Q, Liu Y, Xiao S, Zhang L (2021) Ginsenosides improve nonalcoholic fatty liver disease via integrated regulation of gut microbiota, inflammation and energy homeostasis. Front Pharmacol 12:622841. https://doi.org/10.3389/fphar.2021.622841
Liao Y, Wang X, Huang L, Qian H, Liu W (2023) Mechanism of pyroptosis in neurodegenerative diseases and its therapeutic potential by traditional Chinese medicine. Front Pharmacol 14:1122104. https://doi.org/10.3389/fphar.2023.1122104
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. https://doi.org/10.1038/nature05292
Ling Y, Ding L, Tian Z, Pei L, Wu E (2022) YINDARA-4 relieves visceral hypersensitivity in irritable bowel syndrome rats via regulation of gut microbiota and serotonin levels. Acupunct Herb Med 2(4):274–283. https://doi.org/10.1097/hm9.0000000000000042
Liu B, Piao X, Niu W, Zhang Q, Ma C, Wu T, Gu Q, Cui T, Li S (2020a) Kuijieyuan decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-dependent PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut microbiota. Front Pharmacol 11:1036. https://doi.org/10.3389/fphar.2020.01036
Liu S, Gao J, Zhu M, Liu K, Zhang HL (2020b) Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol 57(12):5026–5043. https://doi.org/10.1007/s12035-020-02073-3
Liu Y, Wang S, Kan J, Zhang J, Zhou L, Huang Y, Zhang Y (2020c) Chinese herbal medicine interventions in neurological disorder therapeutics by regulating glutamate signaling. Curr Neuropharmacol 18(4):260–276. https://doi.org/10.2174/1570159x17666191101125530
Liu S, Long Y, Yu S, Zhang D, Yang Q, Ci Z, Cui M, Zhang Y, Wan J, Li D, Shi A, Li N, Yang M, Lin J (2021a) Borneol in cardio-cerebrovascular diseases: pharmacological actions, mechanisms, and therapeutics. Pharmacol Res 169:105627. https://doi.org/10.1016/j.phrs.2021.105627
Liu T, Song Y, Hu A (2021b) Neuroprotective mechanisms of mangiferin in neurodegenerative diseases. Drug Dev Res 82(4):494–502. https://doi.org/10.1002/ddr.21783
Liu L, Lu Y, Xu C, Chen H, Wang X, Wang Y, Cai B, Li B, Verstrepen L, Ghyselinck J, Marzorati M, Yao Q (2022a) The modulation of Chaihu Shugan formula on microbiota composition in the simulator of the human intestinal microbial ecosystem technology platform and its influence on gut barrier and intestinal immunity in Caco-2/THP1-Blue™ cell co-culture model. Front Pharmacol 13:820543. https://doi.org/10.3389/fphar.2022.820543
Liu Y, Li BG, Su YH, Zhao RX, Song P, Li H, Cui XH, Gao HM, Zhai RX, Fu XJ, Ren X (2022b) Potential activity of traditional Chinese medicine against ulcerative colitis: a review. J Ethnopharmacol 289:115084. https://doi.org/10.1016/j.jep.2022.115084
Liu ZQ, Sun X, Liu ZB, Zhang T, Zhang LL, Wu CJ (2022c) Phytochemicals in traditional Chinese medicine can treat gout by regulating intestinal flora through inactivating NLRP3 and inhibiting XOD activity. J Pharm Pharmacol 74(7):919–929. https://doi.org/10.1093/jpp/rgac024
Liu C, Zhang L, Li Y, Li M, Han H, Wang K (2023a) Traditional Chinese patent medicine in the treatment of Alzheimer’s disease: a systematic review and network meta-analysis. Am J Chin Med 51(3):517–546. https://doi.org/10.1142/s0192415x2350026x
Liu M, Zhang Y, Zhang A, Deng Y, Gao X, Wang J, Wang Y, Wang S, Liu J, Chen S, Yao W, Liu X (2023b) Compound K is a potential clinical anticancer agent in prostate cancer by arresting cell cycle. Phytomedicine 109:154584. https://doi.org/10.1016/j.phymed.2022.154584
Love CJ, Masson BA, Gubert C, Hannan AJ (2022) The microbiota-gut-brain axis in Huntington’s disease. Int Rev Neurobiol 167:141–184. https://doi.org/10.1016/bs.irn.2022.06.005
Lu J, Zhang C, Lv J, Zhu X, Jiang X, Lu W, Lu Y, Tang Z, Wang J, Shen X (2021) Antiallergic drug desloratadine as a selective antagonist of 5HT(2A) receptor ameliorates pathology of Alzheimer’s disease model mice by improving microglial dysfunction. Aging Cell 20(1):e13286. https://doi.org/10.1111/acel.13286
Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF (2019) Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflamm 16(1):53. https://doi.org/10.1186/s12974-019-1434-3
Mancuso C, Santangelo R (2017) Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem Toxicol 107:362–372. https://doi.org/10.1016/j.fct.2017.07.019
Mancuso R, del Valle J, Modol L, Martinez A, Granado-Serrano AB, Ramirez-Núñez O, Pallás M, Portero-Otin M, Osta R, Navarro X (2014) Resveratrol improves motoneuron function and extends survival in SOD1G93A ALS mice. Neurotherapeutics 11(2):419–432. https://doi.org/10.1007/s13311-013-0253-y
Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N (2021) SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc 80(1):37–49. https://doi.org/10.1017/s0029665120006916
Martínez-Solís I, Acero N, Bosch-Morell F, Castillo E, González-Rosende ME, Muñoz-Mingarro D, Ortega T, Sanahuja MA, Villagrasa V (2019) Neuroprotective potential of Ginkgobiloba in retinal diseases. Planta Med 85(17):1292–1303. https://doi.org/10.1055/a-0947-5712
Mayer EA, Nance K, Chen S (2022) The gut-brain axis. Annu Rev Med 73:439–453. https://doi.org/10.1146/annurev-med-042320-014032
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M (2017) The first microbial colonizers of the human gut: composition, activities, and Health implications of the infant gut microbiota. Microbiol Mol Biology Rviews. https://doi.org/10.1128/mmbr.00036-17
Mirza S, Shah K, Patel S, Jain N, Rawal R (2018) Natural compounds as epigenetic regulators of human dendritic cell-mediated Immune function. J Immunother 41(4):169–180. https://doi.org/10.1097/cji.0000000000000201
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW, Morita H, Hattori M, Yamamura T (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 10(9):e0137429. https://doi.org/10.1371/journal.pone.0137429
Morales-Figueroa GG, Pereo-Vega GD, Reyna-Murrieta ME, Pérez-Morales R, López-Mata MA, Sánchez-Escalante JJ, Tapia-Rodriguez MR, Ayala-Zavala JF, Juárez J, Quihui-Cota L (2022) Antibacterial and antioxidant properties of extracts of Yucca baccata, a plant of Northwestern Mexico, against pathogenic bacteria. Biomed Res Int 2022:9158836. https://doi.org/10.1155/2022/9158836
Nuzum ND, Loughman A, Szymlek-Gay EA, Hendy A, Teo WP, Macpherson H (2020) Gut microbiota differences between healthy older adults and individuals with Parkinson’s disease: a systematic review. Neurosci Biobehav Rev 112:227–241. https://doi.org/10.1016/j.neubiorev.2020.02.003
Panza F, Lozupone M, Logroscino G, Imbimbo BP (2019) A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Reviews Neurol 15(2):73–88. https://doi.org/10.1038/s41582-018-0116-6
Peng M, Tabashsum Z, Anderson M, Truong A, Houser AK, Padilla J, Akmel A, Bhatti J, Rahaman SO, Biswas D (2020) Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr Rev Food Sci Food Saf 19(4):1908–1933. https://doi.org/10.1111/1541-4337.12565
Peng Y, Deng X, Yang SS, Nie W, Tang YD (2023) Progress in mechanism of Astragalusmembranaceus and its chemical constituents on multiple sclerosis. Chin Jurnal Integr Med 29(1):89–95. https://doi.org/10.1007/s11655-022-3535-6
Pun J, Chor W (2022) Use of questioning between traditional chinese medicine practitioners and patients to realize TCM Philosophy: holism, five elements and Yin-Yang in the context of doctor-patient communication. Health Commun 37(2):163–176. https://doi.org/10.1080/10410236.2020.1828533
Qiao Y, Liu C, Guo Y, Zhang W, Guo W, Oleksandr K, Wang Z (2022) Polysaccharides derived from Astragalusmembranaceus and Glycyrrhizauralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poult Sci 101(7):101905. https://doi.org/10.1016/j.psj.2022.101905
Qin S, Tang H, Li W, Gong Y, Li S, Huang J, Fang Y, Yuan W, Liu Y, Wang S, Guo Y, Guo Y, Xu Z (2020) AMPK and its activator Berberine in the treatment of neurodegenerative diseases. Curr Pharm Design 26(39):5054–5066. https://doi.org/10.2174/1381612826666200523172334
Radulescu CI, Garcia-Miralles M, Sidik H, Bardile CF, Yusof N, Lee HU, Ho EXP, Chu CW, Layton E, Low D, De Sessions PF, Pettersson S, Ginhoux F, Pouladi MA (2020) Reprint of: manipulation of microbiota reveals altered callosal myelination and white matter plasticity in a model of Huntington disease. Neurobiol Dis 135:104744. https://doi.org/10.1016/j.nbd.2020.104744
Raskov H, Burcharth J, Pommergaard HC, Rosenberg J (2016) Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 7(5):365–383. https://doi.org/10.1080/19490976.2016.1218585
Reitz C, Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88(4):640–651. https://doi.org/10.1016/j.bcp.2013.12.024
Riccio P, Rossano R (2018) Diet, gut microbiota, and vitamins D + A in multiple sclerosis. Neurotherapeutics 15(1):75–91. https://doi.org/10.1007/s13311-017-0581-4
Rutsch A, Kantsjö JB, Ronchi F (2020) The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol 11:604179. https://doi.org/10.3389/fimmu.2020.604179
Sampson TR, Mazmanian SK (2015) Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17(5):565–576. https://doi.org/10.1016/j.chom.2015.04.011
Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, Thron T, Needham BD, Horvath I, Debelius JW, Janssen S, Knight R, Wittung-Stafshede P, Gradinaru V, Chapman M, Mazmanian SK (2020) A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife. https://doi.org/10.7554/eLife.5311
Savaskan E, Mueller H, Hoerr R, von Gunten A, Gauthier S (2018) Treatment effects of Ginkgobiloba extract EGb 761® on the spectrum of behavioral and psychological symptoms of dementia: meta-analysis of randomized controlled trials. Int Psychogeriatr 30(3):285–293. https://doi.org/10.1017/s1041610217001892
Scuderi SA, Ardizzone A, Paterniti I, Esposito E, Campolo M (2020) Antioxidant and anti-inflammatory effect of Nrf2 inducer dimethyl fumarate in neurodegenerative diseases. Antioxidants 9(7):630. https://doi.org/10.3390/antiox9070630
Shabbir U, Arshad MS, Sameen A, Oh DH (2021) Crosstalk between gut and brain in Alzheimer’s disease: the role of gut microbiota modulation strategies. Nutrients 13(2):690. https://doi.org/10.3390/nu13020690
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK (2016) The central nervous system and the gut microbiome. Cell 167(4):915–932. https://doi.org/10.1016/j.cell.2016.10.027
Sonawane SK, Balmik AA, Boral D, Ramasamy S, Chinnathambi S (2019) Baicalein suppresses repeat tau fibrillization by sequestering oligomers. Arch Biochem Biophys 675:108119. https://doi.org/10.1016/j.abb.2019.108119
Song M, Liu Y, Li T, Liu X, Hao Z, Ding S, Panichayupakaranant P, Zhu K, Shen J (2021) Plant natural flavonoids against multidrug resistant pathogens. Adv Sci 8(15):e2100749. https://doi.org/10.1002/advs.202100749
Sonnenburg ED, Sonnenburg JL (2014) Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 20(5):779–786. https://doi.org/10.1016/j.cmet.2014.07.003
Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S (2022) A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin Microbiol Rev 35(1):e0033820. https://doi.org/10.1128/cmr.00338-20
Soto C, Pritzkow S (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 21(10):1332–1340. https://doi.org/10.1038/s41593-018-0235-9
Strandwitz P (2018) Neurotransmitter modulation by the gut microbiota. Brain Res 1693(Pt B):128–133. https://doi.org/10.1016/j.brainres.2018.03.015
Sun HM, Bai LM, Zhang J (2005) Effects of yinxing pingchan recipe and its components on activity of mitochondrial enzyme complex in brain of mice with Parkinson’s disease. Zhongguo Zhong Xi Yi Jie He Za Zhi 25(11):1008–1011
Tang C, Ding R, Sun J, Liu J, Kan J, Jin C (2019) The impacts of natural polysaccharides on intestinal microbiota and immune responses - a review. Food Funct 10(5):2290–2312. https://doi.org/10.1039/c8fo01946k
Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, Costăchescu B, Grumezescu AM, Teleanu RI (2022) An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci 23(11):5938. https://doi.org/10.3390/ijms23115938
Tesco G, Lomoio S (2022) Pathophysiology of neurodegenerative diseases: an interplay among axonal transport failure, oxidative stress, and inflammation? Semin Immunol 59:101628. https://doi.org/10.1016/j.smim.2022.101628
Tian Y, Feng X, Zhou Z, Qin S, Chen S, Zhao J, Hou J, Liu D (2023) Ginsenoside compound K ameliorates osteoarthritis by inhibiting the chondrocyte endoplasmic reticulum stress-mediated IRE1α-TXNIP-NLRP3 axis and pyroptosis. J Agric Food Chem 71(3):1499–1509. https://doi.org/10.1021/acs.jafc.2c06134
Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS (2022) Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci 291:120111. https://doi.org/10.1016/j.lfs.2021.120111
Wang T, Wu Z, Sun L, Li W, Liu G, Tang Y (2018) A computational systems pharmacology approach to investigate molecular mechanisms of herbal formula Tian-Ma-Gou-Teng-Yin for treatment of Alzheimer’s disease. Front Pharmacol 9:668. https://doi.org/10.3389/fphar.2018.00668
Wang Q, Jiang H, Wang L, Yi H, Li Z, Liu R (2019) Vitegnoside mitigates neuronal Injury, mitochondrial apoptosis, and inflammation in an Alzheimer’s disease cell model via the p38 MAPK/JNK pathway. J Alzheimer’s Disease: JAD 72(1):199–214. https://doi.org/10.3233/jad-190640
Wang F, Li R, Tu P, Chen J, Zeng K, Jiang Y (2020) Total glycosides of Cistanchedeserticola promote neurological function recovery by inducing neurovascular regeneration via Nrf-2/Keap-1 pathway in MCAO/R rats. Front Pharmacol 11:236. https://doi.org/10.3389/fphar.2020.00236
Wang J, Wu Q, Ding L, Song S, Li Y, Shi L, Wang T, Zhao D, Wang Z, Li X (2021a) Therapeutic effects and Molecular mechanisms of bioactive compounds against respiratory diseases: traditional Chinese medicine theory and high-frequency use. Front Pharmacol 12:734450. https://doi.org/10.3389/fphar.2021.734450
Wang ZY, Liu J, Zhu Z, Su CF, Sreenivasmurthy SG, Iyaswamy A, Lu JH, Chen G, Song JX, Li M (2021b) Traditional Chinese medicine compounds regulate autophagy for treating neurodegenerative disease: a mechanism review. Biomed Pharmacother 133:110968. https://doi.org/10.1016/j.biopha.2020.110968
Wang H, Shi M, Cao F, Su E (2022a) Ginkgo biloba seed exocarp: a waste resource with abundant active substances and other components for potential applications. Food Res Int 160:111637. https://doi.org/10.1016/j.foodres.2022.111637
Wang J, Chen S, Zhang J, Wu J (2022b) Scutellaria baicalensis georgi is a promising candidate for the treatment of autoimmune diseases. Front Pharmacol 13:946030. https://doi.org/10.3389/fphar.2022.946030
Wang J, Zhu X, Li Y, Guo W, Li M (2022c) Jiedu-Yizhi formula alleviates neuroinflammation in AD rats by modulating the gut microbiota. Evidence-Based Complement Altern Med 2022:4023006. https://doi.org/10.1155/2022/4023006
Wang L, Gou X, Ding Y, Liu J, Wang Y, Wang Y, Zhang J, Du L, Peng W, Fan G (2023a) The interplay between herbal medicines and gut microbiota in metabolic diseases. Front Pharmacol 14:1105405. https://doi.org/10.3389/fphar.2023.1105405
Wang X, Wang Z, Cao J, Dong Y, Chen Y (2023b) Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 11(1):17. https://doi.org/10.1186/s40168-022-01452-3
Wasser CI, Mercieca EC, Kong G, Hannan AJ, McKeown SJ, Glikmann-Johnston Y, Stout JC (2020) Gut dysbiosis in Huntington’s disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun 2(2):fcaa110. https://doi.org/10.1093/braincomms/fcaa110
Woodburn SC, Bollinger JL, Wohleb ES (2021) The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflamm 18(1):258. https://doi.org/10.1186/s12974-021-02309-6
Wu CR, Lin LW, Wang WH, Hsieh MT (2007) The ameliorating effects of LiuWei Dihuang Wang on cycloheximide-induced impairment of passive avoidance performance in rats. J Ethnopharmacol 113(1):79–84. https://doi.org/10.1016/j.jep.2007.05.003
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334(6052):105–108. https://doi.org/10.1126/science.1208344
Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, Star J, Weljie AM, Flint HJ, Metz DC, Bennett MJ, Li H, Bushman FD, Lewis JD (2016) Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65(1):63–72. https://doi.org/10.1136/gutjnl-2014-308209
Wu Q, Cao Y, Liu M, Liu F, Brantner AH, Yang Y, Wei Y, Zhou Y, Wang Z, Ma L, Wang F, Pei H, Li H (2019) Traditional Chinese medicine Shenmayizhi decoction ameliorates memory and cognitive impairment induced by scopolamine via preventing hippocampal cholinergic dysfunction in rats. Neuropsychiatr Dis Treat 15:3167–3176. https://doi.org/10.2147/ndt.S214976
Xia W, Liu B, Tang S, Yasir M, Khan I (2022a) The science behind TCM and gut microbiota interaction-their combinatorial approach holds promising therapeutic applications. Front Cell Infect Microbiol 12:875513. https://doi.org/10.3389/fcimb.2022.875513
Xia Y, Shi H, Qian C, Han H, Lu K, Tao R, Gu R, Zhao Y, Wei Z, Lu Y (2022b) Modulation of gut microbiota by magnesium isoglycyrrhizinate mediates enhancement of intestinal barrier function and amelioration of methotrexate-induced liver injury. Front Immunol 13:874878. https://doi.org/10.3389/fimmu.2022.874878
Xiao H, Sun X, Liu R, Chen Z, Lin Z, Yang Y, Zhang M, Liu P, Quan S, Huang H (2020) Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol Res 151:104559. https://doi.org/10.1016/j.phrs.2019.104559
Xiong W, Zhao X, Xu Q, Wei G, Zhang L, Fan Y, Wen L, Liu Y, Zhang T, Zhang L, Tong Y, Yin Q, Zhang TE, Yan Z (2022) Qisheng Wan formula ameliorates cognitive impairment of Alzheimer’s disease rat via inflammation inhibition and intestinal microbiota regulation. J Ethnopharmacol 282:114598. https://doi.org/10.1016/j.jep.2021.114598
Xu P, Wang H, Li Z, Yang Z (2016) Triptolide attenuated injury via inhibiting oxidative stress in amyloid-beta25-35-treated differentiated PC12 cells. Life Sci 145:19–26. https://doi.org/10.1016/j.lfs.2015.12.018
Xu J, Chen HB, Li SL (2017) Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med Res Rev 37(5):1140–1185. https://doi.org/10.1002/med.21431
Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH, Cui YX, Li J, Ma J, Piao HR, Jin X, Piao LX (2020) Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol 177(22):5224–5245. https://doi.org/10.1111/bph.15261
Yang W, Ip SP, Liu L, Xian YF, Lin ZX (2020) Uncaria rhynchophylla and its major constituents on central nervous system: a review on their pharmacological actions. Curr Vasc Pharmacol 18(4):346–357. https://doi.org/10.2174/1570161117666190704092841
Yu J, Zhang D, Liang Y, Zhang Z, Guo J, Chen Y, Yan Y, Liu H, Lei L, Wang Z, Tang Z, Tang Y, Duan JA (2020) Licorice-Yuanhua herbal pair induces Ileum injuries through weakening epithelial and mucous barrier functions: saponins, flavonoids, and Di-terpenes all involved. Front Pharmacol 11:869. https://doi.org/10.3389/fphar.2020.00869
Zhang Q, Du X, Xu Y, Dang L, Xiang L, Zhang J (2013) The effects of gouqi extracts on Morris maze learning in the APP/PS1 double transgenic mouse model of Alzheimer’s disease. Exp Ther Med 5(5):1528–1530. https://doi.org/10.3892/etm.2013.1006
Zhang M, Zheng HX, Gao YY, Zheng B, Liu JP, Wang H, Yang ZJ, Zhao ZY (2017) The influence of Schisandrin B on a model of Alzheimer’s disease using β-amyloid protein Aβ(1–42)-mediated damage in SH-SY5Y neuronal cell line and underlying mechanisms. J Toxicol Environ Health Part A 80(22):1199–1205. https://doi.org/10.1080/15287394.2017.1367133
Zhang M, Xu L, Yang H (2018) Schisandra Chinensis fructus and its active ingredients as promising resources for the treatment of neurological diseases. Int J Mol Sci 19(7):1970. https://doi.org/10.3390/ijms19071970
Zhang X, Zhang Y, Li R, Zhu L, Fu B, Yan T (2020) Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging 12(10):9405–9426. https://doi.org/10.18632/aging.103215
Zhang B, Zhao J, Guo P, Wang Z, Xu L, Liu A, Du G (2021a) Effects of naodesheng tablets on amyloid beta-induced dysfunction: a traditional Chinese herbal formula with novel therapeutic potential in Alzheimer’s disease revealed by systems pharmacology. Biomed Pharmacother 141:111916. https://doi.org/10.1016/j.biopha.2021.111916
Zhang HY, Tian JX, Lian FM, Li M, Liu WK, Zhen Z, Liao JQ, Tong XL (2021b) Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed Pharmacother 133:110857. https://doi.org/10.1016/j.biopha.2020.110857
Zhang J, Hu K, Di L, Wang P, Liu Z, Zhang J, Yue P, Song W, Zhang J, Chen T, Wang Z, Zhang Y, Wang X, Zhan C, Cheng YC, Li X, Li Q, Fan JY, Shen Y, Han JY, Qiao H (2021c) Traditional herbal medicine and nanomedicine: converging disciplines to improve therapeutic efficacy and human health. Adv Drug Deliv Rev 178:113964. https://doi.org/10.1016/j.addr.2021.113964
Zhang C, Xue P, Zhang H, Tan C, Zhao S, Li X, Sun L, Zheng H, Wang J, Zhang B, Lang W (2022a) Gut brain interaction theory reveals gut microbiota mediated neurogenesis and traditional Chinese medicine research strategies. Front Cell Infect Microbiol 12:1072341. https://doi.org/10.3389/fcimb.2022.1072341
Zhang XB, Guo LP, Zhang W, Tang ZS, Huang LQ (2022b) Geographical views in traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 47(23):6287–6296. https://doi.org/10.19540/j.cnki.cjcmm.20220624.101
Zhang Y, Lin Z, Wang L, Guo X, Hao Z, Li Z, Johnston LJ, Dong B (2022c) Cooperative Interaction of phenolic acids and flavonoids contained in activated charcoal with Herb extracts, involving cholesterol, bile acid, and FXR/PXR activation in broilers fed with mycotoxin-containing diets. Antioxidants. https://doi.org/10.3390/antiox11112200
Zhao T, Tang H, Xie L, Zheng Y, Ma Z, Sun Q, Li X (2019) Scutellaria baicalensis georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol 71(9):1353–1369. https://doi.org/10.1111/jphp.13129
Zhou QZ, Zhang G, Long HB, Lei F, Ye F, Jia XF, Zhou YL, Kang JP, Feng DX (2014) Effect of spinal cord extracts after spinal cord injury on proliferation of rat embryonic neural stem cells and notch signal pathway in vitro. Asian Pac J Trop Med 7(7):562–567. https://doi.org/10.1016/s1995-7645(14)60094-8
Zhou R, He D, Xie J, Zhou Q, Zeng H, Li H, Huang L (2021) The synergistic effects of polysaccharides and ginsenosides from american ginseng (Panaxquinquefolius L.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front Immunol 12:665901. https://doi.org/10.3389/fimmu.2021.665901
Zhu L, Chen Z, Han K, Zhao Y, Li Y, Li D, Wang X, Li X, Sun S, Lin F, Zhao G (2020a) Correlation between mitochondrial dysfunction, cardiovascular diseases, and traditional chinese medicine. Evidence-Based Complement Altern Med 2020:2902136. https://doi.org/10.1155/2020/2902136
Zhu W, Zhou S, Liu J, McLean RJC, Chu W (2020b) Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lyciumbarbarum polysaccharide. Biomed Pharmacother 121:109591. https://doi.org/10.1016/j.biopha.2019.109591
Zhu Z, Liao L, Qiao H (2022) Extracellular vesicle–based drug delivery system boosts phytochemicals’ therapeutic effect for neurodegenerative diseases. Acupunct Herb Med 2(4):229–239. https://doi.org/10.1097/hm9.0000000000000039
Zou YT, Zhou J, Zhu JH, Wu CY, Shen H, Zhang W, Zhou SS, Xu JD, Mao Q, Zhang YQ, Long F, Li SL (2022) Gut microbiota mediates the protective effects of traditional Chinese medicine formula Qiong-Yu-Gao against cisplatin-induced acute kidney injury. Microbiol Spectr 10(3):e0075922. https://doi.org/10.1128/spectrum.00759-22
Acknowledgements
The authors acknowledge BioRender (www.biorender.com, accessed on 16 Jun 2023). Figures in this review were created with the BioRender platform.
Funding
This study was supported by the Natural Science Foundation of Chongqing (cstc2021jcyj-msxm2028), the special project of performance incentive and guidance for scientific research institutions in Chongqing (cstc2022jxjlX0001), the Chongqing Talent Program of Chongqing Municipal People’s Government〔2021〕No. 5, the construction project of the municipal key specialty of Traditional Chinese Medicine of Chongqing Administration of Traditional Chinese Medicine〔2022〕No.4, and the “Xinglin Scholar” Hospital Project of Chengdu University of Traditional Chinese Medicine (YYZX2022150).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guan, Y., Tang, G., Li, L. et al. Herbal medicine and gut microbiota: exploring untapped therapeutic potential in neurodegenerative disease management. Arch. Pharm. Res. 47, 146–164 (2024). https://doi.org/10.1007/s12272-023-01484-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01484-9