Abstract
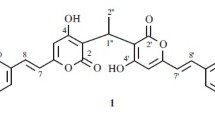
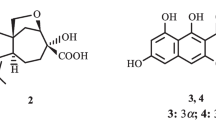
A new β-resorcylic macrolide, 5′-hydroxyzearalenone (1), and six known β-resorcylic macrolides were isolated from the seagrass-derived fungus Fusarium sp. PSU-ES73. Their structures were established by analysis of spectral data. All of the isolated compounds were evaluated for their antibacterial activity against Staphylococcus aureus, both standard and methicillin-resistant strains, as well as their antifungal activity against Cryptococcus neoformans. Only the known compound zearalenone (2) displayed weak antibacterial and antifungal activities.
Similar content being viewed by others
References
Bolliger, G. and Tamm, Ch., Vier neue metabolite von Giberella zeae: 5-formyl-zearalenon, 7′-dehydrozearalenon, 8′-hydroxy- und 8′-epi-hydroxy-zearalenon. Helv. Chim. Acta, 55, 3030–3048 (1972).
Clinical and Laboratory Standards Institute (CLSI). Reference method for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Clinical and Laboratory Standards Institute, Wayne, Pa, (2002a).
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. Clinical and Laboratory Standards Institute, Wayne, Pa, (2002b).
Drummond, A. J. and Waigh, R. D., The development of microbiological methods for phytochemical screening. Recent Research Developments in Phytochemistry, 4, pp. 143–152, (2000).
Fenical, W., Jensen, P. R., and Rowley, D. C., Halovir, an antiviral marine natural product, and derivatives thereof. WO2000035943 (2000).
Folmer, F., Jaspars, M., Dicato, M., and Diederich, M., Photosynthetic marine organisms as a source of anticancer compounds. Phytochem. Rev., 9, 557–579 (2010).
Gillan, F. T., Hogg, R. W., and Drew, E. A., The sterol and fatty acid compositions of seven tropical seagrasses from North Queensland, Australia. Phytochemistry, 23, 2817–2821 (1984).
Kontiza, I., Stavri, M., Zloh, M., Vagias, C., Gibbons, S., and Roussis, V., New metabolites with antibacterial activity from the marine angiosperm Cymodocea nodosa. Tetrahedron, 64, 1696–1702 (2008).
Kornblum, S. S. and Stoopak, S. B., A new tablet disintegrating agent: cross-linked polyvinylpyrrolidone. J. Pharm. Sci., 62, 43–49 (1973).
Ley, S. V. and Burckhardt, S., The use of π-allyltricarbonyliron lactone complexes in the synthesis of the resorcylic macrolides α- and β-zearalenol. J. Chem. Soc. Perkin Trans. I, 3028–3030 (2000).
Miles, C. O., Erasmuson, A. F., Wilkins, A. L., Towers, N. R., Smith, B. L., Garthwaite, I., Scahill, B. G., and Hansen, R. P., Ovine metabolism of zearalenone to α-zearalanol (zeranol). J. Agric. Food Chem., 44, 3244–3250 (1996).
Nichols, P. D. and Johns, R. B., Lipids of the tropical seagrass Thallassia hemprichii. Phytochemistry, 24, 81–84 (1985).
Ohtani, I., Kusumi, T., Kashman, Y., and Kakisawa, H., High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc., 113, 4092–4096 (1991).
Qi, S.-H., Zhang, S., Qian, P.-Y., and Wang, B.-G., Antifeedant, antibacterial, and antilarval compounds from the South China Sea seagrass Enhalus acoroides. Botanica Marina, 51, 441–447 (2008).
Richardson, K. E., Hagler, W. M., and Mirocha, C. J., Production of zearalenone, α- and β-zearalenol, and α- and β- zearalanol by Fusarium spp. in rice culture. J. Agric. Food Chem., 33, 862–866 (1985).
Taub, D., Girotra, N. N., Hoffsommer, R. D., Kuo, C. H., Slates, H. L., Weber, S., and Wendler, N. L., Total synthesis of the macrolide, zearalenone. Tetrahedron, 24, 2443–2461 (1968).
Xiao, Y., Chen, J., Zhang, Y., Shao, Z., and Xu, D., Studies on the chemical constituents of Fusarium sp. from seagrass endophytic fungus. Zhongguo Haiyang Yaowu, 23, 11–13 (2004).
Zhao, L. L., Gai, Y., Kobayashi, H., Hu, C. Q., and Zhang, H. P., 5′-Hydroxyzearalenol, a new β-resorcylic macrolide from Fusarium sp. 05ABR26. Chin. Chem. Lett., 19, 1089–1092 (2008).
Zinedine, A., Soriano, J. M., Moltó, J. C., and Mañes, J., Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem. Toxicol., 45, 1–18 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arunpanichlert, J., Rukachaisirikul, V., Sukpondma, Y. et al. A β-resorcylic macrolide from the seagrass-derived fungus Fusarium sp. PSU-ES73. Arch. Pharm. Res. 34, 1633–1637 (2011). https://doi.org/10.1007/s12272-011-1007-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-1007-1