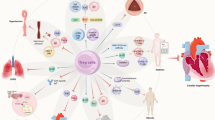
Abstract
Myocardial infarction (MI) remains one of the leading causes of death worldwide. Inflammation and immune responses after MI are of significance to the adverse cardiac remodeling. Regulatory T cells (Tregs) play an important role in suppressing the immune response and thus benefit the post-MI remodeling. After MI, damaged cardiomyocytes may be replaced by scar tissue, leading to systolic and diastolic dysfunction and subsequently adverse remodeling. In this review, we provide an overview of the function and possible mechanisms of Tregs in post-MI heart repair. Specifically, after the occurrence of MI, Tregs infiltrated to peri-infarcted myocardium through CCR5 pathway, CXCR4-CXCL12 axis, and Hippo pathway. Normal functional Tregs can reduce the size of the MI area, improve heart function, and ameliorate myocardial remodeling by inhibiting proinflammatory cells accumulation, changing the proportion of macrophages phenotypes, improving myocardial fibrosis, protecting myocardial cells, and promoting angiogenesis. Eventually, Functional Tregs recruited into the heart can improve MI outcomes. Therefore, targeted therapies with Tregs might provide a promising approach to the treatment of MI remodeling.
Graphical abstract

Similar content being viewed by others
References
Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J : Off Publ Federation Am Soc Exp Biol. 2012;26(6):2253–76. https://doi.org/10.1096/fj.11-193672.
Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259(1):40–59. https://doi.org/10.1111/imr.12170.
Hu W, Wang ZM, Feng Y, Schizas M, Hoyos BE, van der Veeken J, Verter JG, Bou-Puerto R, Rudensky AY. Regulatory T cells function in established systemic inflammation and reverse fatal autoimmunity. Nat Immunol. 2021;22(9):1163–74. https://doi.org/10.1038/s41590-021-01001-4.
Raffin C, Vo LT, Bluestone JA. T cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20(3):158–72. https://doi.org/10.1038/s41577-019-0232-6.
Bluestone JA, Tang Q. T cells-the next frontier of cell therapy. Science (New York, NY). 2018;362(6411):154–5. https://doi.org/10.1126/science.aau2688.
Scholz AS, Handke J, Gillmann H-J, Zhang Q, Dehne S, Janssen H, Arens C, Espeter F, Sander A, Giannitsis E, Uhle F, Weigand MA, Motsch J, Larmann J. Frontline Science: Low regulatory T cells predict perioperative major adverse cardiovascular and cerebrovascular events after noncardiac surgery. J Leukoc Biol. 2020;107(5):717–30. https://doi.org/10.1002/JLB.5HI1018-392RR.
Wang YP, Xie Y, Ma H, Su SA, Wang YD, Wang JA, Xiang MX. Regulatory T lymphocytes in myocardial infarction: a promising new therapeutic target. Int J Cardiol. 2016;203:923–8. https://doi.org/10.1016/j.ijcard.2015.11.078.
Kaplan A, Altara R, Eid A, Booz GW, Zouein FA. Update on the Protective role of regulatory T cells in myocardial infarction: A promising therapy to repair the heart. J Cardiovasc Pharmacol. 2016;68(6):401–13. https://doi.org/10.1097/fjc.0000000000000436.
Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, Frangogiannis NG. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol. 2014;307(8):1233–42.
Weirather J, Hofmann UDW, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115(1):55–67. https://doi.org/10.1161/CIRCRESAHA.115.303895.
Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. https://doi.org/10.1016/j.yjmcc.2013.04.023.
Tang T-T, Yuan J, Zhu Z-F, Zhang W-C, Xiao H, Xia N, Yan X-X, Nie S-F, Liu J, Zhou S-F, Li J-J, Yao R, Liao M-Y, Tu X, Liao Y-H, Cheng X. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107(1):232. https://doi.org/10.1007/s00395-011-0232-6.
Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176(5):2177–87. https://doi.org/10.2353/ajpath.2010.090759.
Wang Y, Dembowsky K, Chevalier E, Stüve P, Korf-Klingebiel M, Lochner M, Napp LC, Frank H, Brinkmann E, Kanwischer A, Bauersachs J, Gyöngyösi M, Sparwasser T, Wollert KC. C-X-C Motif Chemokine receptor 4 blockade promotes tissue repair after myocardial infarction by enhancing regulatory T cell mobilization and immune-regulatory function. Circulation. 2019;139(15):1798–812. https://doi.org/10.1161/CIRCULATIONAHA.118.036053.
Ramjee V, Li D, Manderfield LJ, Liu F, Engleka KA, Aghajanian H, Rodell CB, Lu W, Ho V, Wang T, Li L, Singh A, Cibi DM, Burdick JA, Singh MK, Jain R, Epstein JA. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J Clin Investig. 2017;127(3):899–911. https://doi.org/10.1172/JCI88759.
Xia N, Lu Y, Gu M, Li N, Liu M, Jiao J, Zhu Z, Li J, Li D, Tang T, Lv B, Nie S, Zhang M, Liao M, Liao Y, Yang X, Cheng X. A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction. Circulation. 2020;142(20):1956–73. https://doi.org/10.1161/CIRCULATIONAHA.120.046789.
Zacchigna S, Martinelli V, Moimas S, Colliva A, Anzini M, Nordio A, Costa A, Rehman M, Vodret S, Pierro C, Colussi G, Zentilin L, Gutierrez MI, Dirkx E, Long C, Sinagra G, Klatzmann D, Giacca M. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat Commun. 2018;9(1):2432. https://doi.org/10.1038/s41467-018-04908-z.
Xiao J, Yu K, Li M, Xiong C, Wei Y, Zeng Q. The IL-2/Anti-IL-2 Complex attenuates cardiac ischaemia-reperfusion injury through expansion of regulatory T Cells. Cell Physiol Biochem : Int J Exp Cell Physiol, Biochem Pharmacol. 2017;44(5):1810–27. https://doi.org/10.1159/000485818.
Yan X, Zhang H, Fan Q, Hu J, Tao R, Chen Q, Iwakura Y, Shen W, Lu L, Zhang Q, Zhang R. Dectin-2 deficiency modulates Th1 differentiation and improves wound healing after myocardial infarction. Circ Res. 2017;120(7):1116–29. https://doi.org/10.1161/CIRCRESAHA.116.310260.
Feng W, Li W, Liu W, Wang F, Li Y, Yan W. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol. 2009;87(3):212–8. https://doi.org/10.1016/j.yexmp.2009.06.001.
Xia N, Jiao J, Tang TT, Lv BJ, Lu YZ, Wang KJ, Zhu ZF, Mao XB, Nie SF, Wang Q, Tu X, Xiao H, Liao YH, Shi GP, Cheng X. Activated regulatory T-cells attenuate myocardial ischaemia/reperfusion injury through a CD39-dependent mechanism. Clin Sci (London, England : 197). 2015;128(10):679–93. https://doi.org/10.1042/CS20140672.
Fang J, Hu F, Ke D, Yan Y, Liao Z, Yuan X, Wu L, Jiang Q, Chen L. N, N-dimethylsphingosine attenuates myocardial ischemia-reperfusion injury by recruiting regulatory T cells through PI3K/Akt pathway in mice. Basic Res Cardiol. 2016;111(3):32. https://doi.org/10.1007/s00395-016-0548-3.
Jia D, Jiang H, Weng X, Wu J, Bai P, Yang W, Wang Z, Hu K, Sun A, Ge J. Interleukin-35 promotes macrophage survival and improves wound healing after myocardial infarction in mice. Circ Res. 2019;124(9):1323–36. https://doi.org/10.1161/CIRCRESAHA.118.314569.
Yang K, Zhang Y, Xu C, Li X, Li D. mTORC1 signaling is crucial for regulatory T cells to suppress macrophage-mediated inflammatory response after acute myocardial infarction. Immunol Cell Biol. 2016;94(3):274–84. https://doi.org/10.1038/icb.2015.88.
Zeng Z, Yu K, Chen L, Li W, Xiao H, Huang Z. interleukin-2/anti-interleukin-2 immune complex attenuates cardiac remodeling after myocardial infarction through expansion of regulatory T cells. J Immunol Res. 2016;2016:8493767. https://doi.org/10.1155/2016/8493767.
Hu H, Wu J, Cao C, Ma L. Exosomes derived from regulatory T cells ameliorate acute myocardial infarction by promoting macrophage M2 polarization. IUBMB Life. 2020;72(11):2409–19. https://doi.org/10.1002/iub.2364.
Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309(9):896–908. https://doi.org/10.1001/jama.2013.1363.
Yokota T, McCourt J, Ma F, Ren S, Li S, Kim TH, Kurmangaliyev YZ, Nasiri R, Ahadian S, Nguyen T, Tan XHM, Zhou Y, Wu R, Rodriguez A, Cohn W, Wang Y, Whitelegge J, Ryazantsev S, Khademhosseini A, Teitell MA, Chiou PY, Birk DE, Rowat AC, Crosbie RH, Pellegrini M, Seldin M, Lusis AJ, Deb A. Type V collagen in scar tissue regulates the size of scar after heart injury. Cell. 2020;182(3):545-562.e23. https://doi.org/10.1016/j.cell.2020.06.030.
Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117(1):52–64. https://doi.org/10.1161/circresaha.117.305990.
Sharir R, Semo J, Shimoni S, Ben-Mordechai T, Landa-Rouben N, Maysel-Auslender S, Shaish A, Entin-Meer M, Keren G, George J. Experimental myocardial infarction induces altered regulatory T cell hemostasis, and adoptive transfer attenuates subsequent remodeling. PLoS ONE. 2014;9(12):e113653. https://doi.org/10.1371/journal.pone.0113653.
Li W, Zhang F, Ju C, Lv S, Huang K. The role of CD27-CD70 signaling in myocardial infarction and cardiac remodeling. Int J Cardiol. 2019;278:210–6. https://doi.org/10.1016/j.ijcard.2018.11.132.
Kwon SP, Hwang B-H, Park E-H, Kim HY, Lee J-R, Kang M, Song SY, Jung M, Sohn HS, Kim E, Kim CW, Lee KY, Oh GC, Choo E, Lim S, Chung Y, Chang K, Kim B-S. Nanoparticle-Mediated blocking of excessive inflammation for prevention of heart failure following myocardial infarction. Small. 2021;17(32):e2101207. https://doi.org/10.1002/smll.202101207.
Chen WCW, Lee BG, Park DW, Kim K, Chu H, Kim K, Huard J, Wang Y. Controlled dual delivery of fibroblast growth factor-2 and Interleukin-10 by heparin-based coacervate synergistically enhances ischemic heart repair. Biomaterials. 2015;72:138–51. https://doi.org/10.1016/j.biomaterials.2015.08.050.
Wang N, Liu C, Wang X, He T, Li L, Liang X, Wang L, Song L, Wei Y, Wu Q, Gong C. Hyaluronic acid oligosaccharides improve myocardial function reconstruction and angiogenesis against myocardial infarction by regulation of macrophages. Theranostics. 2019;9(7):1980–92. https://doi.org/10.7150/thno.31073.
Tang J, Shen Y, Chen G, Wan Q, Wang K, Zhang J, Qin J, Liu G, Zuo S, Tao B, Yu Y, Wang J, Lazarus M, Yu Y. Activation of E-prostanoid 3 receptor in macrophages facilitates cardiac healing after myocardial infarction. Nat Commun. 2017;8:14656. https://doi.org/10.1038/ncomms14656.
Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation. 2019;139(2):206–21. https://doi.org/10.1161/CIRCULATIONAHA.118.036065.
Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, Turka LA, Wells AD, Rathmell JC. Foxp3 and Toll-like receptor signaling balance T cell anabolic metabolism for suppression. Nat Immunol. 2016;17(12):1459–66. https://doi.org/10.1038/ni.3577.
Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–70. https://doi.org/10.1182/blood-2010-07-293795.
Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juścińska J, Owczuk R, Szadkowska A, Witkowski P, Młynarski W, Jarosz-Chobot P, Bossowski A, Siebert J, Trzonkowski P. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153(1):23–30. https://doi.org/10.1016/j.clim.2014.03.016.
Acknowledgements
The graphical illustration was created in BioRender.com.
Funding
This work was supported by the National Nature Scientific Funding of China (No. 82072555, No. 81871858, No. 82172550 and No. 81702240), National Natural Science Foundation of Hunan Province (No. 2021JJ30948), and Chinese Cardiovascular Association-Access fund (2019-CCA-ACCESS-023).
Author information
Authors and Affiliations
Contributions
Yishu Wang drafted the manuscript and performed literature search, Danyan Xu critically revised the work. Chunfang Wang and Li Shen read the draft and gave valuable suggestions. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The manuscript does not contain clinical studies or patient data.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Nicola Smart oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Wang, C., Shen, L. et al. The Role of Regulatory T Cells in Heart Repair After Myocardial Infarction. J. of Cardiovasc. Trans. Res. 16, 590–597 (2023). https://doi.org/10.1007/s12265-022-10290-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10290-5