Abstract
Introduction
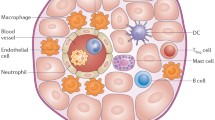
The immune system plays a crucial role in the initiation, development, and resolution of inflammation following myocardial infarction (MI). The lack of oxygen and nutrients causes the death of cardiomyocytes and leads to the exposure of danger-associated molecular patterns that are recognized by the immune system to initiate inflammation.
Results
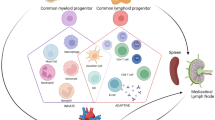
At the initial stage of post-MI inflammation, the immune system further damages cardiac tissue to clear cell debris. The excessive production of reactive oxygen species (ROS) by immune cells and the inability of the anti-oxidant system to neutralize ROS cause oxidative stress that further aggravates inflammation. On the other hand, the cells of both innate and adaptive immune system and their secreted factors are critically instrumental in the very dynamic and complex processes of regulating inflammation and mediating cardiac repair.
Conclusions
It is important to decipher the balance between detrimental and beneficial effects of the immune system in MI. This enables us to identify better therapeutic targets for reducing the infarct size, sustaining the cardiac function, and minimizing the likelihood of heart failure. This review discusses the role of both innate and adaptive immune systems in cardiac tissue damage and repair in experimental models of MI.

Similar content being viewed by others
Abbreviations
- CVD:
-
Cardiovascular diseases
- DAMPs:
-
Damage-associated molecular patterns
- HGF:
-
Hepatocyte growth factor
- HSC:
-
Hematopoietic stem cells
- IRF5:
-
Interferon regulatory factor 5
- MCs:
-
Mast cells
- MI:
-
Myocardial infarction
- MMP:
-
Matrix metalloproteinases
- PRRs:
-
Pattern recognition receptors
- ROS:
-
Reactive oxygen species
- TLR:
-
Toll-like receptors
- Tregs:
-
Regulatory T cells
References
Alwan A, Armstrong T, Bettcher D, Branca F, Chisholm D, Ezzati M, et al. Global status report on noncommunicable diseases 2010: Description of the global burden of NCDs, their risk factors and determinants. Geneva, Switzerland: World Health Organization; 2011.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442.
Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, et al. The global economic burden of noncommunicable diseases. Geneva, Switzerland: World Economic Forum. 2011.
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102.
Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–6.
Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–29.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction from inflammation to fibrosis. Circ Res. 2016;119:91–112.
Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–55.
Mann DL. Innate Immunity and the Failing Heart The Cytokine Hypothesis Revisited. Circ Res. 2015;116:1254–68.
Zlatanova I, Pinto C, Silvestre J-S. Immune modulation of cardiac repair and regeneration: the art of mending broken hearts. Frontiers in cardiovascular medicine. 2016;3:40.
Latet SC, Hoymans VY, Van Herck PL, Vrints CJ. The cellular immune system in the post-myocardial infarction repair process. Int J Cardiol. 2015;179:240–7.
Saparov A, Chen CW, Beckman SA, Wang Y, Huard J. The role of antioxidation and immunomodulation in postnatal multipotent stem cell-mediated cardiac repair. Int J Mol Sci. 2013;14:16258–79.
Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, et al. ERK and p38 MAPK, but not NF-κB, are critically involved in reactive oxygen species–mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;89:661–9.
Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–53.
Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–42.
Stephenson E, Savvatis K, Mohiddin S, Marelli-Berg F. T-cell immunity in myocardial inflammation: pathogenic role and therapeutic manipulation. Br J Pharmacol. 2016. doi: 10.1111/bph.13613.
Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015;116:354–67.
Turner NA. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol. 2016;94:189–200.
Zhang W, Lavine KJ, Epelman S, Evans SA, Weinheimer CJ, Barger PM, et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc. 2015;4:e001993.
Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8:292–300.
Courties G, Moskowitz MA, Nahrendorf M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 2014;71:233–6.
Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, et al. Cutting Edge: IL-1a Is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. Inflammation. 2015;194:499–503.
Lipps C, Nguyen JH, Pyttel L, Lynch TL, Liebetrau C, Aleshcheva G, et al. N-terminal fragment of cardiac myosin binding protein-C triggers pro-inflammatory responses in vitro. J Mol Cell Cardiol. 2016;99:47–56.
Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35.
Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci. 2014;111:16029–34.
Jung K, Kim P, Leuschner F, Gorbatov R, Kim JK, Ueno T, et al. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res. 2013;112:891–9.
Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–39.
Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104.
Li W, Hsiao H-M, Higashikubo R, Saunders BT, Bharat A, Goldstein DR, et al. Heart-resident CCR2 + macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI insight. 2016;1(12):e87315.
Ciz M, Denev P, Kratchanova M, Vasicek O, Ambrozova G, Lojek A. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid Med Cell Longev. 2012;2012:181295.
Klumpe I, Savvatis K, Westermann D, Tschöpe C, Rauch U, Landmesser U, et al. Transgenic overexpression of adenine nucleotide translocase 1 protects ischemic hearts against oxidative stress. J Mol Med (Berl). 2016;94:645–53.
Hafstad AD, Nabeebaccus AA, Shah AM. Novel aspects of ROS signalling in heart failure. Basic Res Cardiol. 2013;108:1–11.
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89.
Kaludercic N, Carpi A, Menabò R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochimica et Biophysica Acta (BBA) Mol Cell Res. 2011;1813:1323–32.
Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009;2:259–69.
Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–90.
Drum BM, Yuan C, Li L, Liu Q, Wordeman L, Santana LF. Oxidative stress decreases microtubule growth and stability in ventricular myocytes. J Mol Cell Cardiol. 2016;93:32–43.
Brasier AR. The nuclear factor-κB–interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–8.
Bonetti P, Lerman L, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75.
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni M, Debuque RJ, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–9.
Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol. 2014;70:74–82.
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis. Circ Res. 2016;118:1021–40.
Dostal D, Glaser S, Baudino TA. Cardiac fibroblast physiology and pathology. Compr Physiol. 2015;5:887–909.
Gerarduzzi C, Di Battista JA. Myofibroblast repair mechanisms post-inflammatory response: a fibrotic perspective. Inflamm Res. 2017;66:451–65.
Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115:284–95.
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47.
Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–46.
Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1066–70.
Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–80.
Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–73.
Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–9.
Grisanti LA, Gumpert AM, Traynham CJ, Gorsky JE, Repas AA, Gao E, et al. Leukocyte-Expressed β2-Adrenergic Receptors are Essential for Survival Following Acute Myocardial Injury. Circulation. 2016;134:153–67.
Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, et al. Myocardial infarction activates CCR2 + hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;16:477–87.
Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37.
Natoli G, Monticelli S. Macrophage activation: glancing into diversity. Immunity. 2014;40:175–7.
Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–37.
Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, et al. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation. 2015;132:1880–90.
Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162:597–605.
Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med. 2015;212:497–512.
Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–82.
Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–8.
Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112:1624–33.
Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–22.
Harel-Adar T, Mordechai TB, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci. 2011;108:1827–32.
Mewhort HE, Lipon BD, Svystonyuk DA, Teng G, Guzzardi DG, Silva C, et al. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-β1. Am J Physiol Heart Circ Physiol. 2016;310:H716–24.
Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, et al. Fibroblast-Specific Genetic Manipulation of p38 MAPK in vivo Reveals its Central Regulatory Role in Fibrosis. Circulation. 2017:CIRCULATIONAHA. 116.026238.
Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187–97.
Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63:1556–66.
Ruparelia N, Godec J, Lee R, Chai JT, Dall’Armellina E, McAndrew D, et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J. 2015;36:1923–34.
Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125:1234–45.
Peng Y, Latchman Y, Elkon KB. Ly6Clow monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182:2777–85.
Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L, et al. Exosomes derived from dendritic cells improve cardiac function via activation of CD4 + T lymphocytes after myocardial infarction. J Mol Cell Cardiol. 2016;91:123–33.
Komarowska I, Coe D, Wang G, Haas R, Mauro C, Kishore M, et al. Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity. 2015;42:1087–99.
Lappegård KT, Garred P, Jonasson L, Espevik T, Aukrust P, Yndestad A, et al. A vital role for complement in heart disease. Mol Immunol. 2014;61:126–34.
De Hoog VC, Timmers L, Van Duijvenvoorde A, De Jager SC, Van Middelaar BJ, Smeets MB, et al. Leucocyte expression of complement C5a receptors exacerbates infarct size after myocardial reperfusion injury. Cardiovasc Res. 2014;103:521–9.
Mueller M, Herzog C, Larmann J, Schmitz M, Hilfiker-Kleiner D, Gessner JE, et al. The receptor for activated complement factor 5 (C5aR) conveys myocardial ischemic damage by mediating neutrophil transmigration. Immunobiology. 2013;218:1131–8.
Emmens RW, Baylan U, Juffermans LJ, Karia RV, Ylstra B, Wouters D, et al. Endogenous C1-inhibitor production and expression in the heart after acute myocardial infarction. Cardiovasc Pathol. 2016;25:33–9.
Ueda Y, Gullipalli D, Song W-C. Modeling complement-driven diseases in transgenic mice: values and limitations. Immunobiology. 2016;221:1080–90.
Pouw R, Vredevoogd D, Kuijpers T, Wouters D. Of mice and men: the factor H protein family and complement regulation. Mol Immunol. 2015;67:12–20.
Ratelade J, Verkman A. Inhibitor (s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica. Mol Immunol. 2014;62:104–13.
Kritikou E, Kuiper J, Kovanen PT, Bot I. The impact of mast cells on cardiovascular diseases. Eur J Pharmacol. 2016;778:103–15.
Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14:478–94.
Ngkelo A, Richart A, Kirk JA, Bonnin P, Vilar J, Lemitre M, et al. Mast cells regulate myofilament calcium sensitization and heart function after myocardial infarction. J Exp Med. 2016;213:1353–74.
Hofmann U, Frantz S. Role of T-cells in myocardial infarction. Eur Heart J. 2016;37:873–9.
Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, et al. Activation of CD4 + T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–63.
Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35.
Moraru M, Roth A, Keren G, George J. Cellular autoimmunity to cardiac myosin in patients with a recent myocardial infarction. Int J Cardiol. 2006;107:61–6.
Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER. Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol. 2005;45:1939–45.
Cheng X, Yu X, Ding Y-j Fu, Q-q Xie J-j, T-t Tang, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97.
Yan X, Shichita T, Katsumata Y, Matsuhashi T, Ito H, Ito K, et al. Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction. J Am Heart Assoc. 2012;1:e004408.
Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2:e60.
Liao Y-H, Xia N, Zhou S-F, Tang T-T, Yan X-X, Lv B-J, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012;59:420–9.
Savvatis K, Pappritz K, Becher PM, Lindner D, Zietsch C, Volk H-D, et al. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ Heart Fail. 2014;7:161–71.
Curato C, Slavic S, Dong J, Skorska A, Altarche-Xifró W, Miteva K, et al. Identification of noncytotoxic and IL-10–producing CD8 + AT2R + T cell population in response to ischemic heart injury. J Immunol. 2010;185:6286–93.
Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63.
Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–87.
Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, et al. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016;13:167–79.
Y-p Wang, Xie Y, Ma H, S-a Su, Y-d Wang, J-a Wang, et al. Regulatory T lymphocytes in myocardial infarction: a promising new therapeutic target. Int J Cardiol. 2016;203:923–8.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87.
Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol. 2014;307:H1233–42.
Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232.
Matsumoto K, Ogawa M, Suzuki J, Hirata Y, Nagai R, Isobe M. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int Heart J. 2011;52:382–7.
Sharir R, Semo J, Shimoni S, Ben-Mordechai T, Landa-Rouben N, Maysel-Auslender S, et al. Experimental myocardial infarction induces altered regulatory T cell hemostasis, and adoptive transfer attenuates subsequent remodeling. PLoS ONE. 2014;9:e113653.
Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, et al. Foxp3 + CD4 + T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67.
Acknowledgements
This work was supported by a grant from Nazarbayev University (A.S.). W.C. was supported by the NIH Ruth L. Kirschstein NRSA postdoctoral fellowship (5T32HL007208).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Andrew Roberts.
Rights and permissions
About this article
Cite this article
Saparov, A., Ogay, V., Nurgozhin, T. et al. Role of the immune system in cardiac tissue damage and repair following myocardial infarction. Inflamm. Res. 66, 739–751 (2017). https://doi.org/10.1007/s00011-017-1060-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1060-4