Abstract
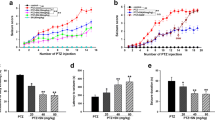
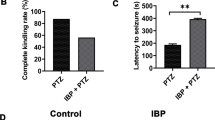
Nerve agents are used in civil wars and terrorist attacks, posing a threat to public safety. Acute exposure to nerve agents such as soman (GD) causes serious brain damage, leading to death due to intense seizures induced by acetylcholinesterase inhibition and neuronal injury resulting from increased excitatory amino-acid levels and neuroinflammation. However, data on the anticonvulsant and neuroprotective efficacies of currently-used countermeasures are limited. Here, we evaluated the potential effects of transient receptor vanilloid 4 (TRPV4) in the treatment of soman-induced status epilepticus (SE) and secondary brain injury. We demonstrated that TRPV4 expression was markedly up-regulated in rat hippocampus after soman-induced seizures. Administration of the TRPV4 antagonist GSK2193874 prior to soman exposure significantly decreased the mortality rate in rats and reduced SE intensity. TRPV4-knockout mice also showed lower incidence of seizures and higher survival rates than wild-type mice following soman exposure. Further in vivo and in vitro experiments demonstrated that blocking TRPV4 prevented NMDA receptor-mediated glutamate excitotoxicity. The protein levels of the NLRP3 inflammasome complex and its downstream cytokines IL-1β and IL-18 increased in soman-exposed rat hippocampus. However, TRPV4 inhibition or deletion markedly reversed the activation of the NLRP3 inflammasome pathway. In conclusion, our study suggests that the blockade of TRPV4 protects against soman exposure and reduces brain injury following SE by decreasing NMDA receptor-mediated excitotoxicity and NLRP3-mediated neuroinflammation. To our knowledge, this is the first study regarding the “dual-switch” function of TRPV4 in the treatment of soman intoxication.








Similar content being viewed by others
References
Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med 2013, 19: 1194–1195.
Chai PR, Hayes BD, Erickson TB, Boyer EW. Novichok agents: a historical, current, and toxicological perspective. Toxicol Commun 2018, 2: 45–48.
McDonough JH Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev 1997, 21: 559–579.
McDonough JH Jr, Shih TM. A study of the N-methyl-D-aspartate antagonistic properties of anticholinergic drugs. Pharmacol Biochem Behav 1995, 51: 249–253.
Shih T, McDonough JH Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci 1999, 6: 86–96.
Skovira JW, McDonough JH, Shih TM. Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci 2010, 40: 56–62.
Olney JW, Collins RC, Sloviter RS. Excitotoxic mechanisms of epileptic brain damage. Adv Neurol 1986, 44: 857–877.
Sloviter RS, Dempster DW. “Epileptic” brain damage is replicated qualitatively in the rat hippocampus by central injection of glutamate or aspartate but not by GABA or acetylcholine. Brain Res Bull 1985, 15: 39–60.
Braitman DJ, Sparenborg S. MK-801 protects against seizures induced by the cholinesterase inhibitor soman. Brain Res Bull 1989, 23: 145–148.
Carpentier P, Foquin-Tarricone A, Bodjarian N, Rondouin G, Lerner-Natoli M, Kamenka JM. Anticonvulsant and antilethal effects of the phencyclidine derivative TCP in soman poisoning. Neurotoxicology 1994, 15: 837–851.
Shih TM, Koenig JA, Acon Chen C. Comparative effects of scopolamine and phencynonate on organophosphorus nerve agent-induced seizure activity, neuropathology and lethality. Toxicol Mech Methods 2019, 29: 322–333.
Sparenborg S, Brennecke LH, Jaax NK, Braitman DJ. Dizocilpine (MK-801) arrests status epilepticus and prevents brain damage induced by soman. Neuropharmacology 1992, 31: 357–368.
Zimmer LA, Ennis M, Shipley MT. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J Comp Neurol 1997, 378: 482–492.
Ferrara-Bowens TM, Chandler JK, Guignet MA, Irwin JF, Laitipaya K, Palmer DD, et al. Neuropathological and behavioral sequelae in IL-1R1 and IL-1Ra gene knockout mice after soman (GD) exposure. Neurotoxicology 2017, 63: 43–56.
Johnson EA, Guignet MA, Dao TL, Hamilton TA, Kan RK. Interleukin-18 expression increases in response to neurovascular damage following soman-induced status epilepticus in rats. J Inflamm (Lond) 2015, 12: 43.
Svensson I, Waara L, Johansson L, Bucht A, Cassel G. Soman-induced interleukin-1 beta mRNA and protein in rat brain. Neurotoxicology 2001, 22: 355–362.
Williams AJ, Berti R, Yao C, Price RA, Velarde LC, Koplovitz I, et al. Central neuro-inflammatory gene response following soman exposure in the rat. Neurosci Lett 2003, 349: 147–150.
Shibasaki K. TRPV4 ion channel as important cell sensors. J Anesth 2016, 30: 1014–1019.
Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by TRP Channels. Neurosci Bull 2018, 34: 120–142.
Bai JZ, Lipski J. Involvement of TRPV4 channels in Aβ40-induced hippocampal cell death and astrocytic Ca2+ signalling. Neurotoxicology 2014, 41: 64–72.
Jie P, Hong Z, Tian Y, Li Y, Lin L, Zhou L, et al. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis 2015, 6: e1775.
Lu KT, Huang TC, Tsai YH, Yang YL. Transient receptor potential vanilloid type 4 channels mediate Na-K-Cl-co-transporter-induced brain edema after traumatic brain injury. J Neurochem 2017, 140: 718–727.
Men C, Wang Z, Zhou L, Qi M, An D, Xu W, et al. Transient receptor potential vanilloid 4 is involved in the upregulation of connexin expression following pilocarpine-induced status epilepticus in mice. Brain Res Bull 2019, 152: 128–133.
Li L, Qu W, Zhou L, Lu Z, Jie P, Chen L, et al. Activation of transient receptor potential vanilloid 4 increases NMDA-activated current in hippocampal pyramidal neurons. Front Cell Neurosci 2013, 7: 17.
Hunt RF, Hortopan GA, Gillespie A, Baraban SC. A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp Neurol 2012, 237: 199–206.
Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci 2007, 27: 1566–1575.
Wang Z, Zhou L, An D, Xu W, Wu C, Sha S, et al. TRPV4-induced inflammatory response is involved in neuronal death in pilocarpine model of temporal lobe epilepsy in mice. Cell Death Dis 2019, 10: 386.
Wang YA, Zhou WX, Li JX, Liu YQ, Yue YJ, Zheng JQ, et al. Anticonvulsant effects of phencynonate hydrochloride and other anticholinergic drugs in soman poisoning: neurochemical mechanisms. Life Sci 2005, 78: 210–223.
Yang J, Fan L, Wang F, Luo Y, Sui X, Li W, et al. Rapid-releasing of HI-6 via brain-targeted mesoporous silica nanoparticles for nerve agent detoxification. Nanoscale 2016, 8: 9537–9547.
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972, 32: 281–294.
Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Green CE, Swezey R, Yang C, et al. Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J Pharmacol Exp Ther 2013, 344: 133–140.
Prager EM, Figueiredo TH, Long RP 2nd, Aroniadou-Anderjaska V, Apland JP, Braga MF. LY293558 prevents soman-induced pathophysiological alterations in the basolateral amygdala and the development of anxiety. Neuropharmacology 2015, 89: 11–18.
Bohnert S, van den Berg RM, Mikler J, Klaassen SD, Joosen MJA. Pharmacokinetics of three oximes in a guinea pig model and efficacy of combined oxime therapy. Toxicol Lett 2020, 324: 86–94.
Li Y, Sun W, Han S, Li J, Ding S, Wang W, et al. IGF-1-involved negative feedback of NR2B NMDA subunits protects cultured hippocampal neurons against NMDA-induced excitotoxicity. Mol Neurobiol 2017, 54: 684–696.
Jie P, Lu Z, Hong Z, Li L, Zhou L, Li Y, et al. Activation of transient receptor potential vanilloid 4 is involved in neuronal injury in middle cerebral artery occlusion in mice. Mol Neurobiol 2016, 53: 8–17.
Zhao H, Zhang K, Tang R, Meng H, Zou Y, Wu P, et al. TRPV4 blockade preserves the blood-brain barrier by inhibiting stress fiber formation in a rat model of intracerebral hemorrhage. Front Mol Neurosci 2018, 11: 97.
Chen X, Yang M, Sun F, Liang C, Wei Y, Wang L, et al. Expression and cellular distribution of transient receptor potential vanilloid 4 in cortical tubers of the tuberous sclerosis complex. Brain Res 2016, 1636: 183–192.
Xu XX, Luo JH. Mutations of N-methyl-D-aspartate receptor subunits in epilepsy. Neurosci Bull 2018, 34: 549–565.
Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science 1989, 244: 1360–1362.
Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science 1991, 254: 1515–1518.
Allen HL, Iversen LL. Phencyclidine, dizocilpine, and cerebrocortical neurons. Science 1990, 247: 221.
Fix AS, Horn JW, Wightman KA, Johnson CA, Long GG, Storts RW, et al. Neuronal vacuolization and necrosis induced by the noncompetitive N-methyl-D-aspartate (NMDA) antagonist MK(+)801 (dizocilpine maleate): a light and electron microscopic evaluation of the rat retrosplenial cortex. Exp Neurol 1993, 123: 204–215.
Chen J, Li Z, Hatcher JT, Chen QH, Chen L, Wurster RD, et al. Deletion of TRPC6 attenuates NMDA receptor-mediated Ca2+ entry and Ca2+-Induced neurotoxicity following cerebral ischemia and oxygen-glucose deprivation. Front Neurosci 2017, 11: 138.
Menigoz A, Ahmed T, Sabanov V, Philippaert K, Pinto S, Kerselaers S, et al. TRPM4-dependent post-synaptic depolarization is essential for the induction of NMDA receptor-dependent LTP in CA1 hippocampal neurons. Pflugers Arch 2016, 468: 593–607.
Liu M, Liu X, Wang L, Wang Y, Dong F, Wu J, et al. TRPV4 inhibition improved myelination and reduced glia reactivity and inflammation in a cuprizone-induced mouse model of demyelination. Front Cell Neurosci 2018, 12: 392.
Acknowledgements
This work was supported by the Special Fund for Military Medical Science (AWS15J007 and BWS16J007) and the National Natural Science Foundation of China (81703505).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., He, H., Long, J. et al. TRPV4 Regulates Soman-Induced Status Epilepticus and Secondary Brain Injury via NMDA Receptor and NLRP3 Inflammasome. Neurosci. Bull. 37, 905–920 (2021). https://doi.org/10.1007/s12264-021-00662-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-021-00662-3