Abstract
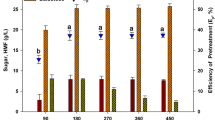
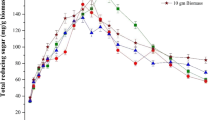
The seaweed has a high content of easily degradable carbohydrates, making it a potential substrate for the production of γ-aminobutyric acid (GABA). In this study, response surface methodology pretreatment and enzymatic saccharification (Es) were conducted on a flask culture of Saccharina japonica seaweed. The optimal hydrolytic conditions were: 10.8% (w/v) slurry content, 0.7% H2SO4, and 121°C for 30 min. Es using enzyme cocktails (Celluclast 1.5 L + Viscozyme L) at 16 U/mL produced 6.26 g/L glucose with an efficiency of 92%. The concentrations of laminarin and fucose (prebiotics) were 10.4 and 0.48 g/L after pretreatment and saccharification, respectively. The suitable monosodium glutamate (MSG) addition was 2% (w/v), and further increase in MSG addition (3–5% (w/v)) had no significant effect on GABA production. The pyridoxal 5′-phosphate (10 µM) addition time of 48–72 h was determined based on the GABA fermentation. Adapted Levilactobacillus brevis KCL010 to high concentrations of mannitol improved the synbiotic fermentation efficiency of S. japonica hydrolysates, further improving the consumption of mixed monosaccharides.
Similar content being viewed by others
References
Lopez-Santamarina, A., J. M. Miranda, A. D. C. Mondragon, A. Lamas, A. Cardelle-Cobas, C. M. Franco, and A. Cepeda (2020) Potential use of marine seaweeds as prebiotics: a review. Molecules 25: 1004.
Charoensiddhi, S., M. A. Conlon, C. M. M. Franco, and W. Zhang (2017) The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 70: 20–33.
Mohd Fauziee, N. A., L. S. Chang, W. A. Wan Mustapha, A. R. Md Nor, and S. J. Lim (2021) Functional polysaccharides of fucoidan, laminaran and alginate from Malaysian brown seaweeds (Sargassum polycystum, Turbinaria ornata and Padina boryana). Int. J. Biol. Macromol. 167: 1135–1145.
Kumar, S. A., M. Magnusson, L. C. Ward, N. A. Paul, and L. Brown (2015) Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar. Drugs 13: 788–805.
Seca, A. M. L. and D. C. G. A. Pinto (2018) Overview of the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar. Drugs 16: 237.
Yang, C.-F., S.-S. Lai, Y.-H. Chen, D. Liu, B. Liu, C. Ai, X.-Z. Wan, L.-Y. Gao, X.-H. Chen, and C. Zhao (2019) Anti-diabetic effect of oligosaccharides from seaweed Sargassum confusum via JNK-IRS1/PI3K signalling pathways and regulation of gut microbiota. Food Chem. Toxicol. 131: 110562.
Xue, M., X. Ji, H. Liang, Y. Liu, B. Wang, L. Sun, and W. Li (2018) The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 9: 1214–1223.
Shin, T., M. Ahn, J. W. Hyun, S. H. Kim, and C. Moon (2014) Antioxidant marine algae phlorotannins and radioprotection: a review of experimental evidence. Acta Histochem. 116: 669–674.
Ferreira, S., A. P. Duarte, M. H. L. Ribeiro, J. A. Queiroz, and F. C. Domingues (2009) Response surface optimization of enzymatic hydrolysis of Cistus ladanifer and Cytisus striatus for bioethanol production. Biochem. Eng. J. 45: 192–200.
Yao, W., L. Yang, Z. Shao, L. Xie, and L. Chen (2020) Identification of salt tolerance-related genes of Lactobacillus plantarum D31 and T9 strains by genomic analysis. Ann. Microbiol. 70: 10.
Doron, S. and D. R. Snydman (2015) Risk and safety of probiotics. Clin. Infect. Dis. 60: S129–S134.
Soccol, C. R., M. R. M. Prado, L. M. B. Garcia, C. Rodrigues, A. B. P. Medeiros, and V. Thomaz-Soccol (2014) Current developments in probiotics. J. Microb. Biochem. Technol. 7: 11–20.
Cui, Y., K. Miao, S. Niyaphorn, and X. Qu (2020) Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int. J. Mol. Sci. 21: 995.
Li, H., T. Qiu, G. Huang, and Y. Cao (2010) Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Fact. 9: 85.
Markowiak, P. and K. Slizewska (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9: 1021.
AOAC (1995) Official Methods of Analysis. 16th ed. Association of Official Analytical Chemists.
Vohra, A. and T. Satyanarayana (2002) Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Process Biochem. 37: 999–1004.
Gajdos, L., V. T. Forsyth, M. P. Blakeley, M. Haertlein, A. Imberty, E. Samain, and J. M. Devos (2021) Production of perdeuterated fucose from glyco-engineered bacteria. Glycobiology 31: 151–158.
Rajauria, G., R. Ravindran, M. Garcia-Vaquero, D. K. Rai, T. Sweeney, and J. O’Doherty (2021) Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 112: 106332.
Hayat, A., T. M. Jahangir, M. Y. Khuhawar, M. Alamgir, A. J. Siddiqui, and S. G. Musharraf (2014) Simultaneous HPLC determination of gamma amino butyric acid (GABA) and lysine in selected Pakistani rice varieties by pre-column derivatization with 2-hydroxynaphth aldehyde. J. Cereal Sci. 60: 356–360.
Kim, N. Y., S.-K. Kim, and C. H. Ra (2021) Evaluation of gamma-aminobutyric acid (GABA) production by Lactobacillus plantarum using two-step fermentation. Bioprocess Biosyst. Eng. 44: 2099–2108.
Haaland, P. D. (1989) Statistical problem solving. pp. 1–18. In: P. D. Haaland (ed.). Experimental Design in Biotechnology. Marcel Dekker.
Laroute, V., R. Mazzoli, P. Loubière, E. Pessione, and M. Cocaign-Bousquet (2021) Environmental conditions affecting GABA production in Lactococcus lactis NCDO 2118. Microorganisms 9: 122.
Shih, Y.-L., S.-C. Hsueh, Y.-L. Chen, J.-S. Chou, H.-Y. Chung, K.-L. Liu, H.-W. Jair, Y.-Y. Chuang, H.-F. Lu, J.-Y. Liu, and J.-G. Chung (2018) Laminarin promotes immune responses and reduces lactate dehydrogenase but increases glutamic pyruvic transaminase in normal mice in vivo. In Vivo 32: 523–529.
Nakata, T., D. Kyoui, H. Takahashi, B. Kimura, and T. Kuda (2016) Inhibitory effects of laminaran and alginate on production of putrefactive compounds from soy protein by intestinal microbiota in vitro and in rats. Carbohydr. Polym. 143: 61–69.
Usov, A. I., G. P. Smirnova, and N. G. Klochkova (2001) Polisakharidy vodorosleĭ. 55. Polisakharidnyĭ sostav nekotorykh burykh vodorosleĭ Kamchatki [Algae polysaccharides. 55. Polysaccharide composition of some brown Kamchatka algae]. Bioorg. Khim. 27: 444–448.
Charoensiddhi, S., M. A. Conlon, M. S. Vuaran, C. M. M. Franco, and W. Zhang (2017) Polysaccharide and phlorotannin-enriched extracts of the brown seaweed Ecklonia radiata influence human gut microbiota and fermentation in vitro. J. Appl. Phycol. 29: 2407–2416.
Kong, Q., S. Dong, J. Gao, and C. Jiang (2016) In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 91: 867–871.
Fu, X., C. Cao, B. Ren, B. Zhang, Q. Huang, and C. Li (2018) Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 183: 230–239.
Ham, S., S. K. Bhatia, R. Gurav, Y.-K. Choi, J.-M. Jeon, J.-J. Yoon, K.-Y. Choi, J. Ahn, H. T. Kim, and Y.-H. Yang (2022) Gamma aminobutyric acid (GABA) production in Escherichia coli with pyridoxal kinase (pdxY) based regeneration system. Enzyme Microb. Technol. 155: 109994.
Villegas, J. M., L. Brown, G. Savoy de Giori, and E. M. Hebert (2016) Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT 67: 22–26.
Yunes, R. A., E. U. Poluektova, M. S. Dyachkova, K. M. Klimina, A. S. Kovtun, O. V. Averina, V. S. Orlova, and V. N. Danilenko (2016) GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 42: 197–204.
Yang, S.-Y., F.-X. Lü, Z.-X. Lu, X.-M. Bie, Y. Jiao, L.-J. Sun, and B. Yu (2008) Production of gamma-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids 34: 473–478.
Kim, J.-H., S. P. Shoemaker, and D. A. Mills (2009) Relaxed control of sugar utilization in Lactobacillus brevis. Microbiology (Reading) 155: 1351–1359.
Choe, M., H. Min, Y.-H. Park, Y.-R. Kim, J.-S. Woo, and Y.-J. Seok (2019) Structural insight into glucose repression of the mannitol operon. Sci. Rep. 9: 13930.
Choe, M., Y.-H. Park, C.-R. Lee, Y.-R. Kim, and Y.-J. Seok (2017) The general PTS component HPr determines the preference for glucose over mannitol. Sci. Rep. 7: 43431.
Kanagachandran, K., P. F. Stanbury, S. J. Hall, and A. Ishizaki (1997) Glucose repression of xylose utilization by Lactococcus lactis IO-1. Biotechnol. Lett. 19: 923–925.
Zhang, Y. and P. V. Vadlani (2015) Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 119: 694–699.
Cui, F., Y. Li, and C. Wan (2011) Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 102: 1831–1836.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No.2022R1F1A1074594).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, S.Y., Ra, C.H. Evaluation of Pretreatment and GABA Production Using Levilactobacillus brevis Fermentation of the Seaweed Saccharina japonica. Biotechnol Bioproc E 28, 568–576 (2023). https://doi.org/10.1007/s12257-023-0073-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-023-0073-9