Abstract
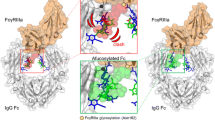
The clinical use of therapeutic antibodies has increased sharply because of their many advantages over conventional small molecule drugs, particularly with respect to their affinity, specificity, and serum stability. Tumor or infected cells are removed by the binding of antibody Fc regions to Fc gamma receptors (FcγRs), which stimulate the activation of immune effector cells. Aglycosylated full-length IgG antibodies expressed in bacteria have different Fc conformations compared to their glycosylated counterparts produced in mammalian cells. As a result, they are unable to bind FcγRs, resulting in little to no activation of immune effector cells. In this study, we created a combinatorial library randomized at the upper CH2 loops of an aglycosylated Fc variant (Fc5: E382V/M428) and used a high-throughput flow cytometry library screening method, combined with bacterial display of homodimeric Fc domains for enhanced FcγR binding affinity. The trastuzumab Fc variant containing the identified mutations (Q295R, L328W, A330V, P331A, I332Y, E382V, M428I) not only exhibited over 120 fold higher affinity of specific binding to FcγRI than wild type aglycosylated Fc, but also retained pH-dependent FcRn binding. These results show that an aglycosylated antibody expressed in bacteria can be evolved for novel FcγR affinity and specificity.
Similar content being viewed by others
References
Reichert, J. M. (2012) Marketed therapeutic antibodies compendium. MAbs. 4: 413–415.
Ryu, J., H. Kim, and D. Nam (2012) Current status and perspectives of biopharmaceutical drugs. Biotechnol. Bioproc. Eng. 17: 900–911.
Hogarth, P. M. and G. A. Pietersz (2012) Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat. Rev. Drug Discov. 11: 311–331.
Jung, S. (2013) Tailoring immunoglobulin Fc for highly potent and serum-stable therapeutic antibodies. Biotechnol. Bioproc. Eng. 18: 625–636.
Jung, S. T., T. H. Kang, and G. Georgiou (2010) Efficient expression and purification of human aglycosylated Fcγ receptors in Escherichia coli. Biotechnol. Bioeng. 107: 21–30.
Nimmerjahn, F. and J. V. Ravetch (2008) Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8: 34–47.
Jung, S. T., S. T. Reddy, T. H. Kang, M. J. Borrok, I. Sandlie, P. W. Tucker, and G. Georgiou (2010) Aglycosylated IgG variants expressed in bacteria that selectively bind FcγRI potentiate tumor cell killing by monocyte-dendritic cells. Proc. Natl. Acad. Sci. USA. 107: 604–609.
Bruhns, P., B. Iannascoli, P. England, D. A. Mancardi, N. Fernandez, S. Jorieux, and M. Daëron (2009) Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113: 3716–3725.
Lu, J., J. L. Ellsworth, N. Hamacher, S. W. Oak, and P. D. Sun (2011) Crystal structure of Fcγ receptor I and its implication in high affinity γ-immunoglobulin binding. J. Biol. Chem. 286: 40608–40613.
Pfefferkorn, L. C. and M. W. Fanger (1989) Cross-linking of the high affinity Fc receptor for human immunoglobulin G1 triggers transient activation of NADPH oxidase activity. Continuous oxidase activation requires continuous de novo receptor cross-linking. J. Biol. Chem. 264: 14112–14120.
Bevaart, L., J. Goldstein, L. Vitale, C. Russoniello, J. Treml, J. Zhang, R. F. Graziano, J. H. W. Leusen, J. G. J. Winkel, and T. Keler (2006) Direct targeting of genetically modified tumour cells to FcγRI triggers potent tumour cytotoxicity. Brit. J. Haematol. 132: 317–325.
van der Poel, C. E., R. M. Spaapen, J. G. van de Winkel, and J. H. Leusen (2011) Functional characteristics of the high affinity IgG receptor, FcγRI. J. Immunol. 186: 2699–2704.
Cohen-Solal, J. F., L. Cassard, W. H. Fridman, and C. Sautes-Fridman (2004) Fc gamma receptors. Immunol. Lett. 92: 199–205.
Yeung, Y. A., M. K. Leabman, J. S. Marvin, J. Qiu, C. W. Adams, S. Lien, M. A. Starovasnik, and H. B. Lowman (2009) Engineering human IgG1 affinity to human neonatal Fc receptor: Impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 182: 7663–7671.
Kuo, T. T. and V. G. Aveson (2011) Neonatal Fc receptor and IgG-based therapeutics. MAbs. 3: 422–430.
Roopenian, D. C. and S. Akilesh (2007) FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7: 715–725.
Valdés, R., A. Tamayo, M. González, S. Padilla, D. Geada, W. Ferro, L. Milá, L. Gómez, R. Alemán, A. Leyva, C. García, O. Mendoza, T. Alvarez, L. Dorta, Y. Villega, D. Cecilia, H. Aragón, T. González, M. La O, and J. López (2012) Production of a monoclonal antibody by ascites, hollow fiber system, and transgenic plants for vaccine production using CB.Hep-1 mAb as a study case. Biotechnol. Bioproc. Eng. 17: 145–159.
Du, Z., M. Mujacic, K. Le, G. Caspary, H. Nunn, C. Heath, and P. Reddy (2013) Analysis of heterogeneity and instability of stable mAb-expressing CHO cells. Biotechnol. Bioproc. Eng. 18: 419–429.
Chames, P., M. V. Regenmortel, E. Weiss, and D. Baty (2009) Therapeutic antibodies: Successes, limitations and hopes for the future. Brit. J. Pharmacol. 157: 220–233.
Simmons, L. C., D. Reilly, L. Klimowski, T. S. Raju, G. Meng, P. Sims, K. Hong, R. L. Shields, L. A. Damico, P. Rancatore, and D. G. Yansura (2002) Expression of full-length immunoglobulins in Escherichia coli: Rapid and efficient production of aglycosylated antibodies. J. Immunol. Methods. 263: 133–147.
Kipriyanov, S. M. and M. Little (1999) Generation of recombinant antibodies. Mol. Biotechnol. 12: 173–201.
Jeong, K. J., S. H. Jang, and N. Velmurugan (2011) Recombinant antibodies: Engineering and production in yeast and bacterial hosts. Biotechnol. J. 6: 16–27.
Lee, Y. J., H. S. Kim, A. J. Ryu, and K. J. Jeong (2013) Enhanced production of full-length immunoglobulin G via the signal recognition particle (SRP)-dependent pathway in Escherichia coli. J. Biotechnol. 165: 102–108.
Borrok, M. J., S. T. Jung, T. H. Kang, A. F. Monzingo, and G. Georgiou (2012) Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem. Biol. 7: 1596–1602.
Jung, S. T., W. Kelton, T. H. Kang, D. T. W. Ng, J. T. Andersen, I. Sandlie, C. A. Sarkar, and G. Georgiou (2012) Effective phagocytosis of low Her2 tumor cell lines with engineered, aglycosylated IgG displaying high FcγRIIa affinity and selectivity. ACS Chem. Biol. 8: 368–375.
Berntzen, G., E. Lunde, M. Flobakk, J. T. Andersen, V. Lauvrak, and I. Sandlie (2005) Prolonged and increased expression of soluble Fc receptors, IgG and a TCR-Ig fusion protein by transiently transfected adherent 293E cells. J. Immunol. Methods. 298: 93–104.
Kabat, E. A., T. T. Wu, H. M. Perry, K. S. Gottesman, and C. Foeller (1991) Sequences of Proteins of Immunological Interest. U.S. Dept. of Health and Hum. Serv., Bethesda.
Kawarasaki, Y., K. E. Griswold, J. D. Stevenson, T. Selzer, S. J. Benkovic, B. L. Iverson, and G. Georgiou (2003) Enhanced crossover SCRATCHY: Construction and high-throughput screening of a combinatorial library containing multiple nonhomologous crossovers. Nucleic Acids Res. 31: e126.
Andersen, J. T., J. Dee Qian, and I. Sandlie (2006) The conserved histidine 166 residue of the human neonatal Fc receptor heavy chain is critical for the pH-dependent binding to albumin. Eur. J. Immunol. 36: 3044–3051.
Jung, S. T., T. H. Kang, W. Kelton, and G. Georgiou (2011) Bypassing glycosylation: Engineering aglycosylated full-length IgG antibodies for human therapy. Curr. Opin. Biotechnol. 22: 858–867.
Raghavan, M. and P. J. Bjorkman (1996) Fc receptors and their interactions with immunoglobulins. Annu. Rev. Cell Dev. Biol. 12: 181–220.
Ober, R. J., C. Martinez, X. Lai, J. Zhou, and E. S. Ward (2004) Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proc. Natl. Acad. Sci. USA. 101: 11076-110–1.
Dall’Acqua, W. F., P. A. Kiener, and H. Wu (2006) Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J. Biol. Chem. 281: 23514–23524.
Sazinsky, S. L., R. G. Ott, N. W. Silver, B. Tidor, J. V. Ravetch, and K. D. Wittrup (2008) Aglycosylated immunoglobulin G1 variants productively engage activating Fc receptors. Proc. Natl. Acad. Sci. USA. 105: 20167–20172.
Kalergis, A. M. and J. V. Ravetch (2002) Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J. Exp. Med. 195: 1653–1659.
Boruchov, A. M., G. Heller, M.-C. Veri, E. Bonvini, J. V. Ravetch, and J. W. Young (2005) Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Invest. 115: 2914–2923.
Smith, P., D. J. DiLillo, S. Bournazos, F. Li, and J. V. Ravetch (2012) Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc. Natl. Acad. Sci. USA. 109: 6181–6186.
Roopenian, D. C., G. J. Christianson, and T. J. Sproule (2010) Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol. Biol. 602: 93–104.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, S.T., Kang, T.H. & Kim, Di. Engineering an aglycosylated Fc variant for enhanced FcγRI engagement and pH-dependent human FcRn binding. Biotechnol Bioproc E 19, 780–789 (2014). https://doi.org/10.1007/s12257-013-0432-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-013-0432-z