Summary
This short review illustrates the benefits of a multidisciplinary team approach, especially when it comes to the treatment of patients with colorectal cancer liver metastasis. Therefore, the classification to resectable and primarily unresectable disease has to be determined prior to the first treatment decision. Particularly the use of conversion chemotherapy has the potential of altering initially unresectable liver metastasis to a potentially resectable disease. The three possible therapy choices for synchronously metastasized colorectal cancer will be reflected in this review, as well as local therapeutic alternatives or combinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is one of the most common cancers worldwide. Fifty percent of patients with colorectal cancer (CRC) will develop liver metastasis, of which about 10% are synchronously metastasized [1]. Synchronous liver metastasis, which are already present at the time of diagnosis, are considered prognostically less favorable than metachronous metastases [2, 3].
Until a few decades ago, the treatment of metastatic CRC was primarily of palliative intent. With improved chemotherapeutic agents and various local therapies, including resection of metastatic sites, this has changed considerably [4].
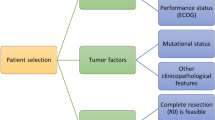
Nowadays, a multidisciplinary team (MDT) approach from diagnosis onwards is essential for the best possible treatment of these patients, which is specifically essential in patients with liver metastasis, where a wide variety of treatment options exists [5]. These range from chemotherapy (neoadjuvant, conversion, adjuvant [so-called perioperative]; given intravenously or intra-arterially) with various combinations and the addition of antibodies dependent upon molecular markers, to surgical resection (mostly in a parenchymal-sparing fashion; performed open, laparoscopic or robotic) and local thermal therapies (e.g., radiofrequency or microwave ablation or stereotactic radiotherapy) (Fig. 1; [3, 6].
Surgical resection of liver metastasis remains the primary treatment intention if the tumors are regarded as resectable in a MDT discussion that includes a hepatobiliary surgical specialist [1].
Important points for the therapy decision and therefore for the MDT discussion is the extent of the disease including the number of metastatic sites and the presence of the primary tumor (including its potential symptomatology), the general health of the patient (ECOG) as well as the molecular biology of the tumor.
Staging is best performed using MRI of the liver including liver-specific contrast in addition to the routine computed tomography (CT) to assess the extent of the liver metastasis [3]. As part of the initial evaluation, FDG-PET can be used, especially if extrahepatic disease has to be excluded [7].
Nowadays synchronous disease detection has become increasingly common and therefore primary intention and sequence of therapies is essential in the MDT decision. If the disease is primarily resectable, the decision has to be made whether a neoadjuvant approach should succeed resection or initial resection should be followed by additional chemotherapy or the resectable disease should simply be resected without chemotherapy.
A decision for neoadjuvant chemotherapy, with a suggested duration of 2 months, should be made depending on prognostic factors: disease-free interval after primary tumor removal, sidedness of the primary lesion, synchronous disease, bilobar hepatic disease, molecular marker [8, 9].
Treatment options for the sum of predictive factors are depicted in several guidelines (NCCN, ESMO) [3, 10, 11].
Thus, prior to the first treatment decision, resectability has to be determined as discussed in the following sections.
Resectable vs. unresectable CRC liver metastasis
Classification into resectable and primarily nonresectable liver metastasis is essential, provided that there are no extrahepatic nonresectable metastases. However, despite multiple recommendations, there is still no standardized classification into these groups.
The best current unresectability criteria are depicted in the multi-institutional CAIRO5 study performed in the Netherlands: expected failure of achieving a complete (R0) resection of all lesions in one single surgical procedure (excluding preOP hypertrophy procedures of the future liver remnant) by surgical resection alone (excluding additional thermal destruction of metastasis: radiofrequency ablation [RFA]/microwave ablation [MWA]), leaving a minimum remnant liver volume of 30% in normal livers, or 40% in compromised livers (chemotherapy pretreated, cirrhotic) [12]. Therefore, number and size or location of metastasis does not define resectability anymore and the future liver remnant (FLR), adequate liver function, and comorbidities are crucial parameters which have to be taken into consideration when discussing therapeutic options within a MDT board [2, 3].
Barely around 20% of the synchronous colorectal liver metastasis patients have initially resectable metastasis. Hence, the larger cohort of newly diagnosed patients have initially unresectable metastasis [13]. These patients may benefit from conversion chemotherapy with the option of altering their initial unresectable liver metastasis to a potentially resectable disease.
Systemic therapy to induce resectability
The optimal combination of available therapies is dependent upon molecular markers and sidedness of the primary. Promising results have been achieved with the combination of triplets together with either EGFR or VEGF antibodies if response of the metastasis is the primary aim [14, 15]. To mention some combinations in detail, according to the ESMO Guidelines, oxaliplatin is recommended for unclear prognostic situations or unfavorable prognostic criteria. Depending on the side of the primary anti-EGFR antibodies are used in left-sided RAS wildtype tumors, whereas folfoxiri/bevacizumab in right-sided and/or RAS mutant tumors [8].
Rediscussion of these patients in a follow-up MDT meeting is, however, key as achieving resectability should be captured as soon as possible because best response always happens within the first months of therapy and the function of the remnant liver deteriorates with every unnecessary additional cycle of chemotherapy [16].
The two main chemotherapeutic agents have specific side effects which interfere with perioperative liver function: therapy including irinotecan induces a steatotic and inflamed liver, the so-called “yellow liver,” also summarized as CASH (chemotherapy-associated steatohepatitis). Conversely the sinusoidal obstruction syndrome (SOS) or the so-called “blue liver” is induced by oxaliplatin, which additionally leads to thrombocytopenia, abnormal liver function, and portal hypertension [17, 18].
The duration of preoperative chemotherapy and its damage to the liver potentially increases the postoperative morbidity and mortality after liver surgery. Therefore, it is important to keep preoperative chemotherapy as short as possible and leave additional treatment for the postoperative phase [4, 17].
The molecular pathological characterization of the tumor has to be known prior to starting first-line therapy. The two important biological markers, RAS and BRAF, determine the treatment combination known to achieve response. In addition, the MSI status needs to be known prior to treatment start, due the fact that immunotherapy leads to favorable response [2, 9].
Evidence for adjuvant chemotherapy especially in resectable disease is low, with recently published negative data from a phase III trial [19].
Liver resection
Liver resection can be performed in various ways and can remove up to 80% of the whole liver volume in a healthy liver without risking postoperative liver insufficiency. When treating liver metastasis, it is however a main goal to preserve as much liver volume as possible, because a necessity of performing a second or third liver resection arises frequently and can only be performed if enough liver volume with adequate in- and outflow is preserved. Techniques of liver resection have evolved considerably over the past few decades and in a specialized hepato-pancreato-biliary unit morbidity and mortality of liver resection for colorectal liver metastasis can be kept very low.
Techniques involve both strategic management in, for example, synchronous disease as well as a combination of interventional procedures and surgery in high volume disease (portal vein embolization/hepatic vein occlusion to induce hypertrophy of a too small future liver remnant or the combination of interventional tumor destruction [RFA, MWA] and liver resection) [3, 20].
Prognostic variable which are associated with poor outcome are synchronous disease, a large primary tumor with positive lymph nodes, short disease-free interval, multiple/bilateral tumors, and a high preoperative carcinoembryonic antigen [21].
Two-stage operation (liver first vs. primary first) vs. simultaneous resection in synchronous mCRC
Irrespective of primarily resectable metastasis or after conversion chemotherapy, there are three possible options resecting synchronously metastasized CRC: liver first, primary first or simultaneous surgery [22, 23]. If conversion chemotherapy including antibody therapy was used a waiting period of 3–5 weeks (3 after chemotherapy alone, 3–4 after chemotherapy + EGFR-AB, 5 after chemotherapy + bevacizumab) [8] after last administration should be kept in mind to help the liver to fully recover and avoid postoperative liver dysfunction [3, 22].
In an analysis of over 7000 synchronously metastatic CRC patients from the LivermetSurvey Registry, the liver-first approach demonstrated prolonged survival for patients with major liver involvement. When using liver first surgery, the patient should be asymptomatic from his primary, severe tumor bleeding or subileus symptomatology should be ruled out. After the patient has recovered from his liver surgery, usually after about 4 weeks, the primary resection can be performed [22].
Simultaneous resection is a one-stage operation of the liver metastasis and the primary tumor in a single operation. For this resection, there are some limitations in terms of anticipated higher complication rates. Therefore, patients should be selected carefully, regarding certain comorbidities as advanced age, the location and the difficulty of resecting the primary (rectal, left or right hemicolon), and most importantly, the extent of the liver resection required; major liver resection (resection of more than three segments) and primary resection should be avoided, due to a high risk of anastomotic leakage especially if the bowel microbiome has been altered due to prolonged chemotherapy [2, 24].
In a meta-analysis of nearly 11,000 patients treated with one of the three surgical options, liver-first showed equal survival, despite higher metastatic burden, as the primary-first approach. However, simultaneous surgery showed a higher morbidity and 30-day mortality. Accordingly, the indication has to be made individually for each patient [25].
Local therapeutic alternatives or combinations with surgical resection
When metastasis are deeply located within the liver requiring a major resection or one has already been performed and additional removal of metastasis would risk a remnant liver volume that is too small, thermal ablative methods have become alternatives: radiofrequency ablation (RFA) or nowadays more often used microwave ablation (MWA) enable good local control when performed by interventional experts [26, 27].
Recurrence of liver metastasis
After completion of initial treatment and radiological tumor clearance, follow-up begins and should include computer tomography and tumor marker control every 3 months for the first 2 years as recurrence is seen frequently within this period; follow-up can be extended to every 6 months after 2 years and to yearly after 5 years [7].
If recurrence is detected MDT decision has to be made similar to initial diagnosis answering essentially whether another potential curative approach is possible. As mentioned above, repeat liver resection is possible if sufficient liver volume is present and can be offered several times [28,29,30].
Take home message
Liver metastasis from colorectal cancer has a variety of potential curative surgical treatment options, which should always be attempted involving a hepatobiliary surgical specialist. When initially deemed unresectable, rediscussion of such patients is key after 2 months of systemic conversion therapy. Multidisciplinary teams lead to the best therapeutic management.
References
Zhou H, Liu Z, Wang Y, Wen X, Amador EH, Yuan L, Ran X, Xiong L, Ran Y, Chen W, Wen Y. Colorectal liver metastasis: molecular mechanism and interventional therapy. Sig Transduct Target Ther. 2022;7(1):70.
German Guideline Program in Oncology (German Cancer Society, German Cancer Aid). S3-Guideline colorectal cancer, long version 2.1, AWMF registrationnumber: 021-007OL. 2019. http://www.leitlinienprogramm-onkologie.de/leitlinien/kolorektales-karzinom/. Accessed 31 Aug 2022.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D’Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Kow AWC. Hepatic metastasis from colorectal cancer. J Gastrointest Oncol. 2019;10(6):1274–98.
Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3(1):50–6.
Bi Y, Shi X, Ren J, Yi M, Han X, Song M. Transarterial chemoembolization with doxorubicin-loaded beads for inoperable or recurrent colorectal cancer. Abdom Radiol (NY). 2021;46(6):2833–8.
Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D. Diagnostic accuracy and impact on management of (18)F-FDG PET and PET/CT in colorectal liver metastasis: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging. 2015;42(1):152–63.
Cervantes A, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022; https://doi.org/10.1016/j.annonc.2022.10.003.
Petrowsky H, Fritsch R, Guckenberger M, De Oliveira ML, Dutkowski P, Clavien PA. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17(12):755–72.
Luzietti E, Pellino G, Nikolaou S, Qiu S, Mills S, Warren O, Tekkis P, Kontovounisios C. Comparison of guidelines for the management of rectal cancer. BJS Open. 2018;2(6):433–51.
Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–59.
Huiskens J, van Gulik TM, van Lienden KP, Engelbrecht MR, Meijer GA, van Grieken NC, Schriek J, Keijser A, Mol L, Molenaar IQ, Verhoef C, de Jong KP, Dejong KH, Kazemier G, Ruers TM, de Wilt JH, van Tinteren H, Punt CJ. Treatment strategies in colorectal cancer patients with initially unresectable liver-only metastases, a study protocol of the randomised phase 3 CAIRO5 study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. 2015;15:365.
Kitano Y, Hayashi H, Matsumoto T, Kinoshita S, Sato H, Shiraishi Y, Nakao Y, Kaida T, Imai K, Yamashita YI, Baba H. Borderline resectable for colorectal liver metastases: Present status and future perspective. World J Gastrointest Surg. 2021;13(8):756–63.
Modest DP, Martens UM, Riera-Knorrenschild J, Greeve J, Florschütz A, Wessendorf S, Ettrich T, Kanzler S, Nörenberg D, Ricke J, Seidensticker M, Held S, Buechner-Steudel P, Atzpodien J, Heinemann V, Seufferlein T, Tannapfel A, Reinacher-Schick AC, Geissler M. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol. 2019;37(35):3401–11.
Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX‑6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702–8.
Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243(1):1–7.
Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200(6):845–53.
Zhu C, Ren X, Liu D, Zhang C. Oxaliplatin-induced hepatic sinusoidal obstruction syndrome. Toxicology. 2021;460:152882.
Kanemitsu Y, Shimizu Y, Mizusawa J, Inaba Y, Hamaguchi T, Shida D, Ohue M, Komori K, Shiomi A, Shiozawa M, Watanabe J, Suto T, Kinugasa Y, Takii Y, Bando H, Kobatake T, Inomata M, Shimada Y, Katayama H, Fukuda H. Hepatectomy followed by mFOLFOX6 versus hepatectomy alone for liver-only metastatic colorectal cancer (JCOG0603): A phase II or III randomized controlled trial. J Clin Oncol. 2021;39(34):3789–99.
Heil J, Schadde E. Simultaneous portal and hepatic vein embolization before major liver resection. Langenbecks Arch Surg. 2021;406(5):1295–305.
Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13(10):1261–8.
Giuliante F, Viganò L, De Rose AM, Mirza DF, Lapointe R, Kaiser G, Barroso E, Ferrero A, Isoniemi H, Lopez-Ben S, Popescu I, Ouellet JF, Hubert C, Regimbeau JM, Lin JK, Skipenko OG, Ardito F, Adam R. Liver-first approach for synchronous colorectal metastases: analysis of 7360 patients from the livermetsurvey registry. Ann Surg Oncol. 2021;28(13):8198–208.
Kambakamba P, Hoti E, Cremen S, Braun F, Becker T, Linecker M. The evolution of surgery for colorectal liver metastases: A persistent challenge to improve survival. Surgery. 2021;170(6):1732–40.
Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey JN, Påhlman L. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41(9):729–41.
Ghiasloo M, Pavlenko D, Verhaeghe M, Van Langenhove Z, Uyttebroek O, Berardi G, Troisi RI, Ceelen W. Surgical treatment of stage IV colorectal cancer with synchronous liver metastases: A systematic review and network meta-analysis. Eur J Surg Oncol. 2020;46(7):1203–13.
McEachron KR, Ankeny JS, Robbins A, Altman AM, Marmor S, D’Souza D, Schat R, Spilseth B, Jensen EH. Surgical microwave ablation of otherwise non-resectable colorectal cancer liver metastases: Expanding opportunities for long term survival. Surg Oncol. 2021;36:61–4.
Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr. 2020;9(1):49–58.
Liu W, Liu JM, Wang K, Wang HW, Xing BC. Recurrent colorectal liver metastasis patients could benefit from repeat hepatic resection. BMC Surg. 2021;21(1):327.
Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, Ijzermans JN, Mirza DF, Elias D, Adam R. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21(4):1276–86.
Inoue Y, Fujii K, Kagota S, Tomioka A, Yamaguchi T, Ohama H, Hamamoto H, Ishii M, Osumi W, Tsuchimoto Y, Terazawa T, Ogura T, Masubuchi S, Yamamoto M, Imoto A, Asai A, Komeda K, Fukunishi S, Hirokawa F, Goto M, Tanaka K, Okuda J, Higuchi K, Uchiyama K. The management of recurrence within six months after hepatic resection for colorectal liver metastasis. Dig Surg. 2020;37(4):282–91.
Funding
Open access funding provided by Sigmund Freud Privatuniversität Wien.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.-M. Tschoegl and T. Gruenberger declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tschoegl, MM., Gruenberger, T. Surgical management of liver metastasis from colorectal cancer. memo 16, 31–35 (2023). https://doi.org/10.1007/s12254-022-00868-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00868-9