Abstract
Purpose
To alleviate the harmful effects of radiations during occupational radiology, radiotherapy and diagnosis, radioprotective system with lower toxicity and extended activity is imperative. Trans-resveratrol (RVL) acts through free radical scavenging/antioxidant mechanism to mitigate the radiation-induced damage. But, its poor solubility and fast metabolism impede its efficacy. Thus, encapsulation of RVL into long circulating solid lipid nanoparticle (SLN) is aimed.
Method
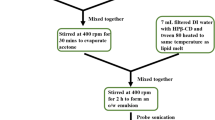
Supercritical CO2 solution of RVL, Gelucire®50/02 and Gelucire®50/13 SLN, was rapidly expanded into aqueous phase containing Tween 80, sonicated and lyophilized to get SLN. Particle size, polydispersity index (PDI), entrapment efficiency (%EE), scanning electron microscopy (SEM), transmission electron microscopy (TEM), differential scanning calorimetry (DSC), X-ray diffraction (XRD), drug release, in vivo pharmacokinetics, antioxidant assays, radiation-induced lipid peroxidation and plasmid DNA relaxation assays were studied. Stability studies were performed to analyze drug degradation and shelf life.
Results
Optimized formulation (F9) had %yield, particle size, PDI, %EE and %drug release (after 72 h) of 68.48 ± 5.73 %, 276.7 ± 5.33 nm, 0.18 ± 0.032, 62.66 ± 4.52 % and 70.05 ± 3.003 %, respectively. Electron microscopy revealed nearly spherical particles, while DSC and XRD showed reduced crystalline peaks. F9 showed higher AUC and sustained release of RVL in rats (i.v. bolus) and increased antioxidant activities and radioprotection as compared to RVL solution. Shelf life of >2 years was predicted for F9 (at 8 °C).
Conclusion
Results are encouraging to use F9 for radiation exposure prophylaxis.










Similar content being viewed by others
Abbreviations
- %EE:
-
Entrapment efficiency
- %IDD:
-
Percentage inhibition of 2-deoxy-d-ribose degradation
- %IDPPH:
-
Percentage DPPH scavenging activity
- %ILPO:
-
Percent inhibition of lipid peroxidation
- %SOR:
-
Superoxide radical scavenging activity
- AA:
-
Antioxidant activity
- CO2 :
-
Carbon dioxide
- DNA:
-
Deoxyribonucleic acid
- DPPH:
-
1,1-Diphenyl-2-Picryl-Hydrazyl Free Radical
- DSC:
-
Differential scanning calorimetry
- IDV:
-
Integrated density values
- ICH:
-
International Council for Harmonisation
- NBT:
-
Nitro blue tetrazolium
- OI:
-
Oxidation index
- PDI:
-
Polydispersity Index
- PEG:
-
Polyethylene glycol
- PMS-NADH:
-
Phenazine methoxy sulphate nicotinamide adenine dinucleotide
- RESS:
-
Rapid expansion of supercritical fluid
- RVL:
-
Trans-resveratrol
- SCF:
-
Supercritical fluid
- SEM:
-
Scanning electron microscopy
- SLNs:
-
Solid lipid nanoparticles
- TEM:
-
Transmission electron microscopy
- XRD:
-
X-ray diffraction
References
Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42(1):21–37.
Hosseinimehr S, Zakaryaee V, Ahmadi A, Akhlaghpoor S. Radioprotective effects of chlorogenic acid against mortality induced by g-irradiation in mice. Methods Find Exp Clin Pharmacol. 2008;30(1):13–6.
Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007;12(19):794–805.
Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, et al. Radioprotection by plant products: present status and future prospects. Phytother Res. 2005;19(1):1–22.
Brown DQ, Pittock III JW, Rubinstein JS. Early results of the screening program for radioprotectors. Int J Radiat Oncol, Biol, Phys. 1982;8(3):565–70.
Martin RF, Broadhurst S, Reum ME, Squire CJ, Clark GR, Lobachevsky PN, et al. In vitro studies with methylproamine A potent new radioprotector. Cancer Res. 2004;64(3):1067–70.
Hahn SM, Krishna MC, DeLuca AM, Coffin D, Mitchell JB. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic Biol Med. 2000;28(6):953–8.
Epperly M, Defilippi S, Sikora C, Gretton J, Kalend A, Greenberger J. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Ther. 2000;7(12):1011–8.
Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng Q-f, Kang SK, Spasojevic I, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–63.
Lachumy SJ, Oon CE, Deivanai S, Saravanan D, Vijayarathna S, Choong YS, et al. Herbal remedies for combating irradiation: a green anti-irradiation approach. Asian Pacific Journal of Cancer Prevention: APJCP. 2013;14(10):5553.
Bhandari PR. Plant products for radioprotection: boon or bane? J Cancer Res Ther. 2013;9(3):545.
Kuntić VS, Stanković MB, Vujić ZB, Brborić JS, Uskoković‐Marković SM. Radioprotectors—the evergreen topic. Chem Biol. 2013;10(10):1791–803.
Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215(1):1–8.
Gescher A, Steward WP, Brown K. Resveratrol in the management of human cancer: how strong is the clinical evidence? Ann N Y Acad Sci. 2013;1290(1):12–20.
Carsten RE, Bachand AM, Bailey SM, Ullrich RL. Resveratrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells. Radiat Res. 2008;169(6):633–8. doi:10.1667/rr1190.1.
Aziz MH, Afaq F, Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem Photobiol. 2005;81(1):25–31. doi:10.1562/2004-08-13-ra-274.
Gülçin İ. Antioxidant properties of resveratrol: a structure–activity insight. Innovative Food Sci Emerg Technol. 2010;11(1):210–8.
Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi:10.1111/j.1749-6632.2010.05842.x.
Singh A, Ahmad I, Akhter S, Jain GK, Iqbal Z, Talegaonkar S, et al. Nanocarrier based formulation of Thymoquinone improves oral delivery: stability assessment, in vitro and in vivo studies. Colloids Surf B: Biointerfaces. 2013;102:822–32.
Singh A, Ahmad I, Akhter S, Zaki Ahmad M, Iqbal ZJ, Ahmad F. Thymoquinone: major molecular targets, prominent pharmacological actions and drug delivery concerns. Curr Bioact Compd. 2012;8(4):334–44.
Pardeshi C, Rajput P, Belgamwar V, Tekade A, Patil G, Chaudhary K, et al. Solid lipid based nanocarriers: an overview. Acta Pharm. 2012;62(4):433–72. doi:10.2478/v10007-012-0040-z.
Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014;15(6):1527–34. doi:10.1208/s12249-014-0177-9.
Battaglia L, Gallarate M, Panciani PP, Ugazio E, Sapino S, Peira E et al. Techniques for the preparation of solid lipid nano and microparticles. In: Sezer AD (ed). Application of Nanotechnology in Drug Delivery. InTech; 2014. doi:10.5772/58405.
Sun Y. Supercritical fluid particle design for poorly water-soluble drugs (review). Curr Pharm Des. 2014;20(3):349–68.
Martin A, Cocero MJ. Micronization processes with supercritical fluids: fundamentals and mechanisms. Adv Drug Deliv Rev. 2008;60(3):339–50. doi:10.1016/j.addr.2007.06.019.
Ahmad I, Akhter S, Ahmad MZ, Shamim M, Rizwi MA, Ahmad FJ. Collagen loaded nano-sized surfactant based dispersion for topical application: formulation development, characterization and safety study. Pharmaceut Dev Tech. 2013;19(4):460–7. doi:10.3109/10837450.2013.795167.
Sánchez-Moreno CA, Larrauri J, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int. 1999;32(6):407–12. http://dx.doi.org/10.1016/S0963-9969(99)00097-6.
Liu F, Ooi VEC, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997;60(10):763–71. http://dx.doi.org/10.1016/S0024-3205(97)00004-0.
Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165(1):215–9.
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi:10.1038/1811199a0.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Guideline IHT. Stability testing of new drug substances and products. Q1A (R2), Current Step 2003;4. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073369.pdf. Accessed 06 March 2013.
Guideline IHT. Validation of analytical procedures: text and methodology Q2 (R1)(2005). Website: www.ich.org/cache/compo/363-272-1 html. 2010.
Urban-Morlan Z, Ganem-Rondero A, Melgoza-Contreras LM, Escobar-Chavez JJ, Nava-Arzaluz MG, Quintanar-Guerrero D. Preparation and characterization of solid lipid nanoparticles containing cyclosporine by the emulsification-diffusion method. Int J Nanomedicine. 2010;5:611–20. doi:10.2147/IJN.S12125.
Date AA, Vador N, Jagtap A, Nagarsenker MS. Lipid nanocarriers (GeluPearl) containing amphiphilic lipid Gelucire 50/13 as a novel stabilizer: fabrication, characterization and evaluation for oral drug delivery. Indian patent application number 2167/MUM/2008. Nanotechnology. 2011;22(27):275102.
Ramalingam P, Ko YT. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf B: Biointerfaces. 2016;139:52–61.
Thies C, Ribeiro Dos Santos I, Richard J, VandeVelde V, Rolland H, Benoit JP. A supercritical fluid-based coating technology 1: process considerations. J Microencapsul. 2003;20(1):87–96.
Ribeiro Dos Santos I, Thies C, Richard J, Le Meurlay D, Gajan V, VandeVelde V, et al. A supercritical fluid-based coating technology. 2: solubility considerations. J Microencapsul. 2003;20(1):97–109.
Singh A, Ahmad I, Ahmad S, Iqbal Z, Ahmad FJ. A novel monolithic controlled delivery system of resveratrol for enhanced hepatoprotection: nanoformulation development, pharmacokinetics and pharmacodynamics. Drug Dev Ind Pharm 2016;1–13. doi:10.3109/03639045.2016.1151032
Park JW, Yun JM, Lee ES, Youn YS, Kim KS, Oh YT, et al. A nanosystem for water-insoluble drugs prepared by a new technology, nanoparticulation using a solid lipid and supercritical fluid. Arch Pharm Res. 2013;36(11):1369–76. doi:10.1007/s12272-013-0187-2.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1):1–10.
Chattopadhyay P, Shekunov BY, Yim D, Cipolla D, Boyd B, Farr S. Production of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx system. Adv Drug Deliv Rev. 2007;59(6):444–53.
Chauhan H, Mohapatra S, Munt DJ, Chandratre S, Dash A. Physical-chemical characterization and formulation considerations for solid lipid nanoparticles. AAPS PharmSciTech. 2016;17(3):640–51. doi:10.1208/s12249-015-0394-x.
Kathe N, Henriksen B, Chauhan H. Physicochemical characterization techniques for solid lipid nanoparticles: principles and limitations. Drug Dev Ind Pharm. 2014;40(12):1565–75. doi:10.3109/03639045.2014.909840.
Kumbhar DD, Pokharkar VB. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: physicochemical investigations. Colloids Surf A Physicochem Eng Asp. 2013;416:32–42.
Dodiya S, Chavhan S, Korde A, Sawant KK. Solid lipid nanoparticles and nanosuspension of adefovir dipivoxil for bioavailability improvement: formulation, characterization, pharmacokinetic and biodistribution studies. Drug Dev Ind Pharm. 2013;39(5):733–43.
Li R, Eun JS, Lee M-K. Pharmacokinetics and biodistribution of paclitaxel loaded in pegylated solid lipid nanoparticles after intravenous administration. Arch Pharm Res. 2011;34(2):331–7.
Acknowledgments
The first author acknowledges the University Grants Commission, New Delhi, India, for the financial support under the Special Assistance Program (SAP-II, 3-58/2011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
In vivo pharmacokinetic study was performed after proper authorization from Institutional Animal Ethics Committee, Jamia Hamdard, New Delhi, and strict guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, Ministry of Culture, and Government of India) were followed.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ahmad, I., Anwar, M., Akhter, S. et al. Supercritical Fluid Technology-Based Trans-Resveratrol SLN for Long Circulation and Improved Radioprotection. J Pharm Innov 11, 308–322 (2016). https://doi.org/10.1007/s12247-016-9254-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-016-9254-9