Abstract
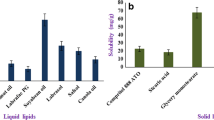
In an effort to develop an alternative formulation of paclitaxel (PTX) suitable for intravenous administration, PTX-loaded sterically stabilized solid lipid nanoparticles (SLNs) were prepared and their pharmacokinetics and biodistribution were investigated. The pegylated SLNs were comprised of trimyristin (TM) as a solid lipid core and egg phosphatidylcholine and pegylated phospholipid as stabilizers. The prepared pegylated TM-SLNs containing PTX exhibited monodispersed size distribution with 217.4 ± 32.8 nm of mean diameter and 99% of distribution was smaller than 556.2 ± 89.9 nm. After PTX in the pegylated TM-SLNs or commercial product, Taxol®, was intravenously administered into femoral vein of rats, concentrations of PTX in plasma and organs such as liver, spleen, kidney, heart and lung were analyzed by HPLC following liquid extraction. Plasma profile of PTX for pegylated TM-SLNs was similar to that for Taxol®, with no statistically significant difference at each time point, although mean plasma levels of PTX at each point tended to be slightly lower in pegylated TM-SLNs than in Taxol®. PTX in the pegylated TM-SLNs was taken up mainly into reticuloendothelial system showing 8-fold and 3-fold higher levels in liver and spleen, respectively, 8 h after administration compared to PTX in Taxol®. Meanwhile, PTX levels in kidney, heart and lung were not different between two formulations. There were no statistically significant differences in pharmacokinetic parameters. Taken together the results, the pegylated TM-SLNs provided similar circulation compared with commercial formulation, Taxol®.
Similar content being viewed by others
References
Chen, D. B., Yang, T. Z., Lu, W. L., and Zhang, Q., In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem. Pharm. Bull., 49, 1444–1447 (2001).
Devalapally, H., Chakilam, A., and Amiji, M. M., Role of nanotechnology in pharmaceutical product development. J. Pharm. Sci, 96, 2547–2565 (2007).
Dobrovolskaia, M. A., Aggarwal, P., Hall, J. B., and McNeil, S. E., Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm, 5, 487–495 (2008).
Fang, J., Nakamura, H., and Maeda, H., The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. doi:10.1016/j.addr.2010.04.009 (2010).
Fundar, A., Cavalli, R., Bargoni, A., Vighetto, D., Zara, G. P., and Gasco, M. R., Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol. Res, 42, 337–343 (2000).
Gao, Y., Gu, W., Chen, L., Xu, Z., and Li, Y., The role of daidzeinloaded sterically stabilized solid lipid nanoparticles in therapy for cardio-cerebrovascular diseases. Biomaterials, 29, 129–136 (2008).
Gelderblom, H., Verweij, J., Nooter, K., and Sparreboom, A., Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer, 37, 1590–1598 (2001).
Lee, M. K., Lim, S. J., and Kim, C. K., Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials, 28, 2137–2146 (2007).
Nornoo, A. O. and Chow, D. S., Cremophor-free intravenous microemulsions for paclitaxel II. Stability, in vitro release and pharmacokinetics. Int. J. Pharm, 349, 117–123 (2008).
Reddy, L. H., Vivek, K., Bakshi, N., and Murthy, R. S., Tamoxifen citrate loaded solid lipid nanoparticles (SLN): preparation, characterization, in vitro drug release, and pharmacokinetic evaluation. Pharm. Dev. Technol, 11, 167–177 (2006).
Sanjula, B., Shah, F. M., Javed, A., and Alka, A., Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J. Drug Target, 17, 249–256. (2009).
Singla, A. K., Garg, A., and Aggarwal, D., Paclitaxel and its formulations. Int. J. Pharm., 20, 179–192 (2002).
Souto, E. B. and Doktorovova, S., Solid lipid nanoparticle formulations: pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzymol, 464, 105–129 (2009).
Sparreboom, A., Scripture, C. D., Trieu, V., Williams, P. J., De, T., Yang, A., Beals, B., Figg, W. D., Hawkins, M., and Desai, N., Comparative preclinical and clinical pharmacokinetics of a cremophor-free nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in cremophor (Taxol). Clin. Cancer Res, 11, 4136–4143 (2005).
Wall, E. M. and Wani, M. C., Camptothecin and Taxol; discovery to clinic-thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res, 55, 753–760 (1993).
Wang, Y. and Wu, W., In situ evading of phagocytic uptake of stealth solid lipid nanoparticles by mouse peritoneal macrophages. Drug Deliv., 13, 189–192 (2006).
Weiss, R. B., Donehower, R. C., Wiernik, P. H., Ohnuma, T., Gralla, R. J., Trump, D. L., Baker, J. R. Jr., Van Echo, D. A., Von Hoff, D. D., and Leyland-Jones, B., Hypersensitivity reactions from taxol. J. Clin. Oncol., 8, 1263–1268 (1990).
Yang, S. C., Lu, L. F., Cai, Y., Zhu, J. B., Liang, B. W., and Yang, C. Z., Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Release, 59, 299–307 (1999).
Zhang, X., Pan, W., Gan, L., Zhu, C., Gan, Y., and Nie, S., Preparation of a dispersible PEGylate nanostructured lipid carriers (NLC) loaded with 10-hydroxycamptothecin by spray-drying. Chem. Pharm. Bull, 56, 1645–1650 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, R., Eun, J.S. & Lee, MK. Pharmacokinetics and biodistribution of paclitaxel loaded in pegylated solid lipid nanoparticles after intravenous administration. Arch. Pharm. Res. 34, 331–337 (2011). https://doi.org/10.1007/s12272-011-0220-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-0220-2