Abstract
The expansion of many wetland species is a function of both clonal propagation and sexual reproduction. The production of ramets through clonal propagation enables plants to move and occupy space near parent ramets, while seeds produced by sexual reproduction enable species to disperse and colonize open or disturbed sites both near and far from parents. The balance between clonal propagation and sexual reproduction is known to vary with plant density but few studies have focused on reproductive allocation with density changes in response to global climate change. Schoenoplectus americanus is a widespread clonal wetland species in North America and a dominant species in Chesapeake Bay brackish tidal wetlands. Long-term experiments on responses of S. americanus to global change provided the opportunity to compare the two modes of propagation under different treatments. Seed production increased with increasing shoot density, supporting the hypothesis that factors causing increased clonal reproduction (e.g., higher shoot density) stimulate sexual reproduction and dispersal of genets. The increase in allocation to sexual reproduction was mainly the result of an increase in the number of ramets that flowered and not an increase in the number of seeds per reproductive shoot, or the ratio between the number of flowers produced per inflorescence and the number of flowers that developed into seeds. Seed production increased in response to increasing temperatures and decreased or did not change in response to increased CO2 or nitrogen. Results from this comparative study demonstrate that plant responses to global change treatments affect resource allocation and can alter the ability of species to produce seeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global change impacts submersed and emergent plant communities globally (Short et al. 2016). To understand the dispersion and establishment of tidal wetland species, it is important to examine their responses to changing climate conditions. Several studies have focused on the responses of tidal wetland species to changes in environmental factors. Gabler et al. (2017) showed that mangroves respond mostly to temperature, CO2, and changes in hydrology. Nehring and Hesse (2008) suggested that the spread of Spartina angelica, an invasive species, is most likely due to increasing global temperatures. Phragmites australis, a cosmopolitan invasive species, is likely to benefit from global changes (Eller et al. 2017) such as an extended growing season, increased nutrient pollution, and higher concentrations of atmospheric CO2 (Caplan et al. 2015; Mozdzer et al. 2016). While these and other studies have assessed the responses of tidal wetland plant communities and ecosystem-level parameters such as plant biomass or productivity to changing climate conditions, few studies have focused on the reproductive responses of plants to changing environmental conditions.
Expansion of wetland species is a function of both clonal and sexual reproduction. Clonal propagation is an adaptive strategy by which plants produce genetically identical individuals that are spaced apart from each other and exchange resources through belowground tissues such as rhizomes, roots, and stolons (de Kroon and van Groenendael 1997; Cornelissen et al. 2014). Clonality is a successful strategy in flowering plants as demonstrated by the enormous diversity of morphological features associated with clonal species (Klimešová 2018). It enables plants to move and occupy space, often to the exclusion of species (Zedler and Kercher 2004). On the other hand, sexual reproduction is also an important strategy that enables species to colonize open or disturbed sites (Kettenring et al. 2015; Kettenring and Whigham 2018). While the benefits of each type of reproduction are clear, it has been difficult to generalize how the allocation to sexual reproduction in ecologically important clonal plants responds to different environmental conditions (Cornelissen et al. 2014).
Some studies suggested that the allocation of effort to these two propagation modes vary with plant density. It was predicted that sexual reproduction should be favored at low plant densities where potential success of sexual reproduction is higher (Loehle 1987; Newell and Tramer 1978) and decreasing reproductive effort in response to increasing density has been reported (Snell and Burch 1975; Williams et al. 1977; Law et al. 1979; Humphrey and Pyke 1998). In contrast, there is also evidence that seed production at higher densities is an adaptive trait. Giroux and Bédard (1995) found greater seed production associated with higher shoot densities of Scirpus pungens (Schoenoplectus pungens) in brackish tidal wetlands. Similar tendencies were reported in other species such as Tussilago farfara (Ogden 1974; Abrahamson 1975; Holler and Abrahamson 1977; Demetrio et al. 2020). Ikegami et al. (2012) used a lattice modeling approach to predict that the production of seeds at high densities is the most efficient evolutionary strategy.
In this study, we focused on the allocation of resources to sexual reproduction in Schoenoplectus americanus, an abundant wetland clonal C3 plant in the sedge family that has variable resource allocation in response to differences in shoot density. Ikegami (2004) showed that S. americanus produced more flowering shoots and increased inflorescence mass in patches with higher shoot densities. Ikegami did not, however, examine aspects of seed productivity in response to differences in density. Neither has the allocation of resources to sexual reproduction been examined as part of long-term experiments to characterize the species response to differences in CO2 concentration, nitrogen availability, temperature, and sea level rise (Arp and Drake 1991; Langley and Megonigal 2010; White et al. 2012; Langley et al. 2013; Mozdzer et al. 2016; Noyce et al. 2019; Lu et al. 2019; Pastore et al. 2017; Cott et al. 2020; Gabriel et al. 2022). We focused on two questions related to S. americanus sexual reproduction: (i) Does sexual reproductive effort increase with increasing shoot density, and (ii) Does sexual reproductive effort, measured by increased allocation to seed production, vary in response to the global change treatments of elevated CO2, temperature, and nitrogen. We examined these topics by analyzing annual shoot density data from three long-term experiments in combination with measurements of density and allocation to sexual reproduction at the long-term experimental sites.
Methods
Study Site and Species
This study was conducted at the Global Change Research Wetland (GCReW), part of Kirkpatrick Marsh (38° 53′ N, 76° 33′ W), a 22-ha brackish tidal wetland in the Rhode River subestuary of Chesapeake Bay (Fig. 1). The wetland varies in elevation such that inundation occurs in 37% of the area twice per day, 41% once per day, and 20% once daily to twice a month (Holmquist et al. 2021). The experimental area is flooded approximately 40% of the time, the tidal range is 44 cm, and the mean soil salinity ranges from 7.8 to 8.5 ppt (Gabriel et al. 2022). The dominant species are the C3 sedge S. americanus and the C4 grasses Spartina patens (Aiton) Muhl. and Distichlis spicata (L.) Kuntze. Other abundant species are Iva frutescens L., Kosteletzkya virginica (L.) C. Presl ex A. Gray, and Schoenoplectus robustus (Pursh) Soják. The invasive non-native haplotype of Phragmites australis (Cav.) Trin. E Steud. is also present and has increased in abundance in recent decades (Holmquist et al. 2021; McCormick et al. 2010).
Location of the GCReW site on Kirkpatrick marsh (38° 53′ N, 76° 33′ W), a brackish tidal wetland in the Rhode River subestuary of Chesapeake Bay. A Black dots show the distribution of Schoenoplectus americanus. B Enlarged map showing the location of the study area within the Chesapeake Bay. The distribution of S. americanus was provided from GBIF Occurrence Download (https://doi.org/10.15468/dl.qv53hc). The map was created with Quantum Geographic Information System (QGIS) software version 3.16.0 (QGIS Development Team 2002). The base map was obtained from Natural Earth
S. americanus is distributed in tidal wetlands on the coasts of North and South America (Fig. 1; Koyama 1963; Tiner and Burke 1995) and is a dominant or co-dominant plant in GCReW experiments that focus on species and ecosystem responses to elevated CO2, nitrogen (N), and temperature (Drake et al. 1989; Erickson et al. 2007; Pastore et al. 2016, 2017; Langley et al. 2009a, b; Lu et al. 2019; Noyce et al. 2019; Zhu et al. 2022; Gabriel et al. 2022).
CO2 Experiment
This experiment began in 1987 to investigate plant responses to elevated CO2 and was established in three different plant communities (Drake et al. 1989; Drake 1992). One community, hereafter referred to as “C3,” was dominated by S. americanus. The second community, hereafter referred to as “C4,” was dominated by Spartina patens and Distichlis spicata. Both species are in the Poaceae and use the C4 photosynthetic pathway (Ehleringer and Cerling 2002) that responds minimally to elevated CO2 compared to C3 species (Ghannoum et al. 2000). A third community, hereafter referred to as “Mixed,” had all three species. Each community has deviated from the original composition in 1986, generally increasing in dominance of relatively flood-tolerant S. americanus (Gabriel et al. 2022). In each community, there are 5 open-top chambers (ca. 1 m diameter) that continuously receive ambient air and five chambers that receive ambient air + 340 ppm CO2 (the level of CO2 that was chosen when the original experiment was started; it represented a doubling of atmospheric CO2 at that time; Drake 1992) during treatment periods. There are also five non-chamber controls in each community, hereafter referred to as “control plots.” The ambient and elevated treatments run 24 h per day from May 1 to October 31 annually.
CO2 × N Experiment
A second long-term experiment that also uses open-top chambers was initiated in 2006 to investigate plant and ecosystem responses to elevated CO2 and N addition (Langley et al. 2009a, b). The study was established in an area of the Kirkpatrick Marsh that was in the same general location as the CO2 experiment but where the plant community was dominated by S. americanus. Five chambers (ca. 2 m diameter) receive ambient air, five chambers receive elevated CO2 (ambient air + 340 ppm CO2), five chambers receive ambient air and elevated soil nitrogen, and five chambers receive elevated CO2 and N addition. Each chamber has an outside non-chambered control area, hereafter referred to as “control plots.” Chambers that receive CO2 are managed using the same protocols as described above for the CO2 experiment, except that CO2 is added only during daylight hours. Chambers in the N addition treatment are fertilized monthly from May to September with NH4Cl (5 g N m−2 month−1 = 25 g N m−2 year−1), a level chosen to represent a polluted system (Langley and Megonigal 2010).
Warming Experiment
In 2016, the Salt Marsh Accretion Response to Temperature eXperiment (SMARTX) was initiated to investigate plant and ecosystem responses to whole-ecosystem warming using infrared lamps and belowground heating cables (Noyce et al. 2019). We used the 12 experimental plots in the S. americanus-dominated plant community (a.k.a. C3 community) treated with four levels of warming (control, + 1.7 °C, + 3.4 °C, + 5.1 °C) at ambient CO2, with each treatment replicated three times. The temperature range was chosen to cover projected temperatures for the Chesapeake Bay region in 2100. Using four treatment levels allows the researchers to determine if the temperature gradient results in nonlinear responses for the variables measured (Noyce et al. 2019). Thus, replication in the warming experiment (n = 3) was lower than in the other two experiments (n = 5).
Schoenoplectus Density and Sexual Reproduction
During the 2019 growing season (August–September), we counted the number of vegetative and reproductive shoots in each chamber and control plot for each of the three experiments. We randomly harvested 10 flowering shoots from each chamber and plot for determination of reproductive effort (described below). If there were fewer than 10 flowering shoots per chamber or plot, we harvested all of them.
Flowering shoots produce a terminal inflorescence composed of 1–15 spikelets. Each spikelet has one or more flowers that can develop into fruits that are a firm, brown achene. In the laboratory, we counted the number of spikelets on each harvested shoot and dissected each spikelet to determine the number of mature and dispersed fruits. The number of dispersed fruits could be determined because each one left a depression on the spikelet rachilla. Immature fruits on each spikelet, always at the terminal end of a spikelet, were also counted. Immature fruits were much smaller than mature fruits, were not black, and were still subtended by a bract that is not present at the base of mature fruits.
Data Analysis
Statistical analyses were performed using R version 3.6.0 (R Core Team 2019). To evaluate seed reproduction, three sexual reproduction variables were used: (1) flowering ratio (the ratio of the number of flowering shoots to the total number of shoots), (2) potential seed production (sum of mature and immature seeds) per reproductive shoot, and (3) ratio of mature to potential seeds in each reproductive shoot.
To test the hypothesis that sexual reproductive effort increased with increasing shoot density, we performed a logistic regression model for variables 1 and 3, and a linear regression model for variable 2 to examine the association of shoot density and the three reproduction variables. These analyses were performed with the glm and lm functions. We also calculated the main and interactive effects of a factorial combination of shoot density and treatment. In each experiment, shoot count data from control plots and all treatments were pooled and analyzed with the models.
The effects of experimental treatments (CO2, N, temperature) on the three reproductive variables were also examined. Data for the three reproductive variables were tested for normality with the Shapiro–Wilk test (Shapiro.test function in the stats R package version 3.6.0) before and after log transformation. Log transformation was accomplished by applying the equation log(x + 1) to each data set. We used untransformed data on the following analysis because the data were not normally distributed either before or after log transformation (Supplemental Table 1). For the CO2 experiment, the main effects and interactions between treatments (control, ambient CO2, elevated CO2) and communities (C3, C4, Mixed) were analyzed with the Scheirer-Ray-Hare test, a non-parametric test for a two-way factorial design (scheirerRayHare function in the rcompanion R package version 2.3.25) and differences of all pairs of groups were compared with the Steel–Dwass test, a non-parametric test using ranks for multiple comparison. For the CO2 × N and warming experiments, treatments were compared using Kruskal–Wallis test (kruskal.test function in the stats R package version 3.6.0) and differences of all pairs of groups were compared with Steel–Dwass test. We considered p values of < 0.05 to be particularly meaningful but this arbitrary p value threshold was not used as the sole source of inference to judge whether our results were scientifically meaningful (Smith 2020). We also made comparisons of the treatment effect for the three experiments by calculating an absolute difference of the response variables between the treatment chambers with the ambient chambers or + 0 °C plots.
Results
Sexual Reproductive Effort Responses to Differences in Shoot Density in Non-Chambered Control Plots
The flowering ratio increased significantly as shoot density increased in the control plots of all three experiments (Fig. 2A, D, G; Table 1). In the CO2 experiment, the number of potential seeds per reproductive shoot and the ratio of mature seeds to potential seeds increased with increasing shoot density in the control plots (Fig. 2B, C; Table 1). In the CO2 × N experiment, the number of potential seeds was positively related to shoot density, but the relationship was not significant and the ratio of mature to potential seeds was negative in the control plots (Fig. 2E, F; Table 1). There was no relationship between the two seed-related variables and density in the warming experiment control plots (Fig. 2H, I; Table 1).
Logistic and liner regression model relationships between reproductive variables and shoot density in control plots showed that seed production increased with increasing shoot density. The increase in allocation to seed production was mainly caused by an increase in flowering ratio. A–C = CO2 experiment, D–F = CO2 × N experiment, G–I = warming experiment. A, D, G Flowering ratio in each experimental plot. B, E, H Number of potential seeds per reproductive shoot. C, F, I Ratio of mature to potential seeds per reproductive shoot. The plots with deep color indicate overlapping. Black boxes indicate the community type as described in the “Methods” section. Density indicates number of shoots per m2. The asterisks indicate significant relationship between shoot density and reproductive variables at α = 0.05
Sexual Reproduction Responses Under Experimental Conditions
CO2 Experiment
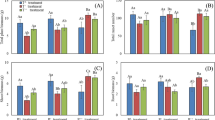
The number of potential seeds per reproductive shoot and the ratio of mature to potential seeds differed sharply among treatments (Table 2). The flowering ratio were not significantly different, but the means were greater in the ambient and elevated chambers (Fig. 3A). The number of potential seeds was higher in the ambient and elevated chambers compared to controls (Fig. 3B). The ratio of mature to potential seeds were also higher in the ambient chambers compared to the controls and elevated chambers, particularly in the elevated chambers (control vs. ambient: p = 0.011, ambient vs. elevated: p = 5.9E-05 in Steel–Dwass test, Fig. 3C). The number of potential seeds and the ratio of mature to potential seeds differed among communities (Table 2), and treatments and communities interacted to affect the ratio of mature to potential seeds (Table 2). The means and standard errors for the three reproductive variables in each plant community are shown in Supplemental Table 2 and Supplemental Fig. 1. The treatment effect of elevated CO2 on all three variables, the number of potential seeds per m2 was negative, and the number of mature seeds per m2 was positive (Fig. 3J, K, L and Supplemental Fig. 2G, H).
Reproductive variables of seed production increased in response to increasing temperatures but decreased or did not change in response to increased CO2 or nitrogen. A–C = CO2 experiment, D–F = CO2 × N experiment, G–I = warming experiment. A, D, G = flowering ratio, B, E, H = number of potential seeds per reproductive shoot, C, F, I = ratio of mature to potential seeds. The error bars indicate the SE. Differences of all pairs of groups were compared using Steel–Dwass test. Values with the same letter indicate no significant difference at α = 0.05. J–L = treatment effect showed by differences between average of treatment plots and ambient or + 0 °C plots
CO2 × N Experiment
There were significant treatment differences for all reproductive variables (Table 2). The flowering ratio were higher in all treatment chambers and highest in ambient chambers (Fig. 3D). The mean number of potential seeds was higher in the treatment chambers compared to controls, but there were no significant differences (Fig. 3E). The mean ratio of mature to potential seeds was more variable, and there were no significant differences between controls and treatment chambers (Fig. 3F). Means and standard errors for three reproductive variables are in Supplemental Table 2. It is difficult to compare the controls and chambers in this experiment because during the summer of 2019, an outbreak of an unknown insect, not apparent in most years, consumed inflorescences inordinately in the control plots (A. Langley, personal observation). The treatment effect of elevated CO2 and nitrogen on all three variables and the number of potential and mature seeds per m2 was negative (Fig. 3J, K, L and Supplemental Fig. 2G, H).
Warming Experiment
There were significant treatment differences for the flowering ratio and the number of potential seeds per reproductive shoot (Table 2). Warming increased the flowering ratio and the number of potential seeds (Fig. 3G, H). The ratio of mature to potential seeds were not significantly different but were greater in all warming plots (Fig. 3I). Means and standard error for three reproductive variables are in Supplemental Table 2. The treatment effect of temperature on all three variables and the number of potential and mature seeds per m2 was positive (Fig. 3J, K, L and Supplemental Fig. 2G, H).
Effects of Experimental Conditions on the Relationship Between Seed Production and Shoot Density
CO2 Experiment
The flowering ratio increased significantly with increasing shoot density in the control plots (Fig. 2A) but there was no statistically significant relationship with density in the ambient chambers (Fig. 4A; Table 1). There was a significantly positive relationship between the flowering ratio and density in the elevated chambers (Fig. 4A; Table 1). The number of potential seeds increased with increasing shoot density in the control plots (Fig. 2B). However, the relationship was significantly negative in the ambient chambers and while the relationship was positive in the elevated chambers it was not significant (Fig. 4B; Table 1). The ratio of mature to potential seeds increased in the controls and treatment chambers (Figs. 2C and 4C; Table 1).
Logistic and liner regression model relationships between reproductive variables and shoot density in experimental conditions showed that no positive relationship between shoot density and flowering ratio under CO2 increased, N addition, and modest warming (+ 1.7 °C) conditions. A–C = CO2 experiment, D–I = CO2 × N experiment, J–L = Warming experiment. A, D, G, J Flowering ratio in each experimental plot. B, E, H, K Number of potential seeds per reproductive shoot. C, F, I, L Ratio of mature to potential seeds per reproductive shoot. The plots with deep color indicate overlapping. Black boxes indicate the community type as described in the “Methods” section. Density indicates number of shoots per m.2. The asterisks after treatment name indicate significant relationship between shoot density and reproductive variables at α = 0.05
CO2 × N Experiment
The flowering ratio was significantly related to shoot density in the control plots (Fig. 2D). The relationship was negative in the ambient chambers (Fig. 4D; Table 1) and there were no significant relationships in the other three treatments (Fig. 4D, G; Table 1). The number of potential seeds was positively related to shoot density, but the relationship was not statistically significant in the control plots (Fig. 2E). The relationships were not significant in treatment chambers (Fig. 4E, H). The ratio of mature to potential seeds had a negative relationship to density in the control chambers (Fig. 2F). However, the ratio increased with increasing shoot density in the ambient and elevated + N chambers (Fig. 4F, I; Table 1).
Warming Experiment
The flowering ratio increased with increasing shoot density in control plots (Fig. 2G) and the + 3.4 °C and + 5.1 °C plots but the relationship was not statistically significant in the + 1.7 °C plots (Fig. 4J; Table 1). The number of potential seeds had no statistically significant relationship to density in any of the elevated temperature plots (Fig. 4K; Table 1). The ratio of mature to potential seeds had no statistically significant relationship to density in control plots (Fig. 2I) but the relationships were negative and statistically significant in the + 1.7 and + 3.4 °C plots and positive and statistically significant in the + 5.1 °C plots (Fig. 4L; Table 1).
Discussion
In over 35 years of experimental research, S. americanus in the Kirkpatrick Marsh has been temporally dynamic with changes in the relative abundance and morphology. The changes have occurred in each of the areas where the long-term experiments have been conducted but especially in the communities where the CO2 experiment is located. The density of S. americanus increased in each of the three communities, at the expense of Spartina patens (Drake 2014; Lu et al. 2019). Lu et al. (2019) found that the diameter and height of ramets of S. americanus decreased over the first 30 years of the experiment, and the results of the present study demonstrate that changes in the density of ramets can influence the reproductive effort of S. americanus. We also found that the relationship between seed production and ramet density may vary depending on experimental conditions. S. americanus is widespread nationally (Fig. 1; Smith 2002), is a dominant species in Chesapeake Bay wetlands (McCormick and Somes 1982), and will be an important species in coastal wetlands as they respond to changing climatic conditions (Noyce et al. 2023; Vahsen et al. 2023). It is an especially important species in influencing wetland response to sea level rise as it is most abundant and productive when tidal flooding is frequent (Kirwan and Guntenspergen 2015; Holmquist et al. 2021).
The long-term GCReW experiments have focused on plant production and density responses in S. americanus, but none of the prior research considered seed production responses, an important component of ecosystem function (e.g., Pearse et al. 2017; Solbreck and Knape 2017). Our results from the non-chambered control plots demonstrate a positive relationship between shoot density and sexual reproductive effort for S. americanus (Fig. 2). The increase of sexual reproductive effort was mainly the result of an increase in the number of ramets that flowered. Studies of other species suggest that the relationship between sexual reproductive effort and density is variable. Winn and Pitelka (1981) suggested that increased shoot density would result in increased competition between ramets and decreased resource availability for seed production, a view supported by Newell and Tramer (1978) and Loehle (1987). In contrast, increased seed production at higher densities may be an adaptive trait in competitive environments as seed production and dispersal would enable species to escape from competitive crowding and colonize new environments (Abrahamson 1975, 1980; Winn and Pitelka 1981; Giroux and Bedard 1995). A feature of clonal species like S. americanus that would support increased seed production, even at higher densities, is division of labor (Roiloa et al. 2014), a process that enables physiologically connected ramets of clonal plants to share resources. In environments such as wetlands where clonal herbaceous species are often dominant and where resources are spatially heterogeneous (e.g., Weiss et al. 2014; Dong et al. 2015), division of labor would enable ramets to secure resources that are locally available with ramets that may be rooted in areas where resources are less available. Ikegami (2004) demonstrated that division of labor occurs in S. americanus and that process may be partially responsible for the positive relationships between density in the non-chambered control plots and the two seed production variables in the CO2 experiment (Fig. 2B, C) as the sharing of resources can benefit a ramet that is in suboptimal conditions. If so, we would expect that more ramets in a population have the resources to flower than in the absence of division of labor, an outcome that is consistent with our results.
In the experimental manipulations, we found that there is almost no positive relationship between shoot density and sexual reproductive effect under increased CO2, N addition, and modest warming (+ 1.7 °C) conditions. Seed production has been predicted or shown to both increase and decrease in crops in response to higher CO2 (Ziska et al. 2001; Allen et al. 2004; Lenka et al. 2017) and the same has been shown for native plants (Billings and Billings 1983; Zangerl and Bazzaz 1984; HilleRisLambers et al. 2009). For S. americanus, when shoot density is not considered, the lower means for all three variables in the elevated chambers (Elev in Fig. 3A–C) indicate that other unknown factors may limit seed production, especially the maturation of seeds. The number of potential seeds per flowering shoot were statistically the same for ambient and elevated chambers (Fig. 3B), but the mean ratio of mature to potential seeds was statistically lower in the elevated chambers, indicating that resources may limit seed maturation under elevated CO2. Multiple studies also have shown that the productivity response of S. americanus to elevated CO2 is nitrogen limited. Langley and Megonigal (2010) showed that N addition enhanced the CO2 stimulation of plant productivity in the first year of the CO2 × N experiment. Lu et al. (2019) combined data from the CO2 × N and CO2 experiments and found that elevated CO2 acting alone caused the diameter and height of S. americanus shoots to decrease, a morphological response that was compensated for in terms of primary production by increased shoot density. They concluded that N limitation caused by elevated CO2 was responsible for the morphological changes as added N reversed the effects in the CO2 × N experiment. The N limitation caused production of smaller individual stems at higher plant density (Lu et al. 2019) and may have restricted seed production at the higher density (Fig. 4A, D). Unfortunately, interpretation of the results from the CO2 × N experiment were complicated by the impacts of an insect outbreak across the GCReW area prior to time we sampled this site. A positive response between shoot density and the number of potential seeds per flowering shoot (Fig. 2E) and a significant decrease in the same variable when density was not considered indicate that CO2 and N decrease the potential for seed production. Resource limitations may have a negative impact of seed maturation (compare Fig. 3E, F), especially in response to increasing shoot densities (Fig. 4E–I).
Temperature has variable effects on seed production in crops and native species (Klady et al. 2011; Caignard et al. 2017; Lenka et al. 2017; Lippmann et al. 2019). Our results demonstrate that temperature will impact sexual reproductive effort in S. americanus. Means for all three variables related to sexual reproductive effort were higher in the + 1.7, + 3.4, and + 5.1 °C treatments and the differences were significant for two of the variables (Fig. 3G–I). A comparison of the treatment effects in all three experiments clearly demonstrate the importance of temperature. All three variables (Fig. 3J, K) were positive in the heated plots of the warming experiment compared to the CO2 and CO2 × N experiments and the same was observed when the number of potential and number of mature seeds per m2 were compared (Supplemental Fig. 2G, H). Data from the CO2 and CO2 × N experiments also provide evidence of a potential temperature effect on sexual reproductive effort. Higher means for the three variables from the ambient treatment in the CO2 and CO2 × N experiments (Amb in Fig. 3A–F) versus the non-chambered control suggests a chamber effect that we interpret to be due to the cumulative effect of slightly higher temperatures in the ambient chambers over many years. Temperature differences inside and outside of chambers have been reported for experiments that are not designed to evaluate temperature (Vanaja et al. 2006; Messerli et al. 2015), and Drake et al. (1989) reported temperatures inside chambers in the CO2 experiment at GCReW to be 1–2 °C higher than outside temperatures. Noyce et al. (2019), using plant density as a variable in the calculation of net primary production, also found annual differences in response to the temperature treatments in the first three years of the SMARTX experiment. They showed that root-to-shoot allocation was changed depending on warming and suggested that modest warming (+ 1.7 °C) caused plant demand for N to outpace the soil N supply while extreme warming (+ 3.4 and + 5.1 °C) caused the N supply to outpace plant N demand. In this study, there is no positive relationship between flowering ratio and plant density in ambient chambers in the CO2 and CO2 × N experiments, and at + 1.7 °C plots in the warming experiment. However, there was a positive relationship at the + 3.4 and + 5.1 °C plots in warming experiment. Seed production of S. americanus may be suppressed under modest warming conditions but not be suppressed under extreme warming conditions.
Few studies have documented the effects of multiple factors on seed production. Miyagi et al. (2007) found that elevated CO2 increased seed production in eleven annual species but concluded that the response was limited by nitrogen availability. Osanai et al. (2017) examined the interacting effects of elevated CO2 and temperature on cotton under variable soil conditions. They found, similar to this study, temperature and elevated CO2 had a positive impact on fruit production, but the temperature response was larger, and the two variables interacted through the effects on nutrient availability, especially nitrogen availability. However, increasing nutrient availability may not result in increased seed production in nutrient-limited ecosystems. Molau and Shaver (1997) found that elevating temperatures increased sexual reproductive effort of Eriophorum vaginatum, a species in the Cyperaceae (similar to S. americanus) in open-topped chambers in arctic environments, but adding N and P to experimental plots had no effect on seed number.
In summary, results of this comparative study demonstrate that an important wetland clonal species produces more flowering ramets in response to increasing density and that increasing temperature has a positive effect on the allocation of resources to sexual reproduction. Globally, the results suggest that clonal tidal wetland species will respond to changes in temperature by producing more seeds that will be dispersed to and colonize new sites created by rising sea levels. We also demonstrate that when other environmental factors (CO2 and N) are considered, increased allocation of resources to sexual reproduction may not result in an increase in the production of more mature seeds, even though reproductive ramets may have produced more flowers (i.e., potential seeds). The results suggest that the adaptative reproductive strategy of S. americanus will be impacted by future climate changes. The study demonstrated that an increased allocation of resources to sexual reproduction but not the production of mature seeds, an important component of the species ecological strategy (Ikegami et al. 2012). Future research on this species and other important wetland clonal species should focus on experiments that include several levels of the environmental factors (especially CO2 and N). The design of the temperature experiment already provides the opportunity to determine if different temperatures influence sexual reproductive effort differently. The research should also consider studies of seed dispersal, similar to those conducted by Kudoh and Whigham (2001) as seedling growth and establishment and the ability of species to colonize suitable habitats will be an important aspect of wetlands in the future.
Data Availability
Data are available from Aoi Kudoh (kudoh.aoi.72x@st.kyoto-u-ac.jp)
References
Abrahamson, W. 1975. Reproductive strategies in dewberries. Ecology 56: 721–726. https://doi.org/10.2307/1935508.
Abrahamson, W. 1980. Demography and vegetative reproduction. In Demography and evolution in plant populations, ed. O.T. Solbrig, 89–106. Oxford: Blackwell Scientific.
Allen, L.H., P.V. Prasad, and R.M. Goodman. 2004. Crop responses to elevated carbon dioxide. Encyclopedia of Plant and Crop Science: 346–348. https://doi.org/10.1081/E-EPCS120005566.
Arp, W.J., and B.G. Drake. 1991. Increased photosynthetic capacity of Scirpus olneyi after 4 years of exposure to elevated CO2. Plant, Cell & Environment 14: 1003–1006. https://doi.org/10.1111/j.1365-3040.1991.tb00971.x.
Billings, W.D., and S.M. Billings. 1983. Growth and reproduction in four populations of Saxifraga flagellaris, a rare arctic-alpine plant species, in controlled environments. Bulletin of the Ecological Society of America 64: 58.
Caignard, T., A. Kremer, C. Firmat, M. Nicolas, S. Venner, and S. Delzon. 2017. Increasing spring temperatures favor oak seed production in temperate areas. Scientific Reports 7: 1–8. https://doi.org/10.1038/s41598-017-09172-7.
Caplan, J.S., R.N. Hager, J.P. Megonigal, and T.J. Mozdzer. 2015. Global change accelerates carbon assimilation by a wetland ecosystem engineer. Environmental Research Letters 10: 115006. https://doi.org/10.1088/1748-9326/10/11/115006.
Cornelissen, J.H.C., Y.B. Song, F.H. Yu, and M. Dong. 2014. Plant traits and ecosystem effects of clonality: A new research agenda. Annals of Botany 114: 369–376. https://doi.org/10.1093/aob/mcu113.
Cott, G.M., M.A.K. Jansen, and J.P. Megonigal. 2020. Uptake of organic nitrogen by coastal wetland plants under elevated CO2. Plant and Soil 450: 521–535. https://doi.org/10.1007/s11104-020-04504-5.
Demetrio, G.R., M.E.A. Barbosa, and F. de F. Coelho. 2020. Plant density influence on life history traits of a perennial herb in rocky outcrops, southeastern Brazil. Iheringia Série Botânica 75: e2020014. https://doi.org/10.21826/2446-82312020v75e2020014.
Dong, B.C., P. Alpert, Q. Zhang, and F.H. Yu. 2015. Clonal integration in homogeneous environments increases performance of Alternanthera philoxeroides. Oecologia 179: 393–403. https://doi.org/10.1007/s00442-015-3338-y.
Drake, B.G., P.W. Leadley, W.J. Arp, D. Nassiry, and P.S. Curtis. 1989. An open top chamber for field studies of elevated atmospheric CO2 concentration on saltmarsh vegetation. Functional Ecology 3: 363–371. https://doi.org/10.2307/2389377.
Drake, B.G. 1992. A field study of the effects of elevated CO2 on ecosystem processes in a Chesapeake Bay wetland. Australian Journal of Botany 40: 579–595. https://doi.org/10.1071/BT9920579.
Drake, B.G. 2014. Rising sea level, temperature, and precipitation impact plant and ecosystem responses to elevated CO2 on a Chesapeake Bay wetland: Review of a 28-year study. Global Change Biology 20: 3329–3343. https://doi.org/10.1111/gcb.12631.
de Kroon, H., and J. van Groenendael. 1997. The Ecology and Evolution of Clonal Plants. Leiden: Backhuys Publishers.
Ehleringer, J.R., and T.E. Cerling. 2002. C3 and C4 photosynthesis. In Encyclopedia of Global Environmental Change, Volume 2, The Earth system: Biological and ecological dimensions of global environmental change, ed. H.A. Mooney and J.G. Canadell, 186–190. Chichester: John Wiley & Sons Ltd.
Eller, F., H. Skálová, J.S. Caplan, G.P. Bhattarai, M.K. Burger, J.T. Cronin, W.-Y. Guo, X. Guo, E.L.G. Hazelton, K.M. Kettenring, C. Lambertini, M.K. McCormick, L.A. Meyerson, T.J. Mozdzer, P. Pyšek, B.K. Sorrell, D.F. Whigham, and H. Brix. 2017. Cosmopolitan species as models for ecophysiological responses to global change: The common reed Phragmites australis. Frontiers Plant Science 8: 1833. https://doi.org/10.3389/fpls.2017.01833.
Erickson, J.E., J.P. Megonigal, G. Peresta, and B.G. Drake. 2007. Salinity and sea level mediate elevated CO2 effects on C3–C4 plant interactions and tissue nitrogen in a Chesapeake Bay tidal wetland. Global Change Biology 13: 202–215. https://doi.org/10.1111/j.1365-2486.2006.01285.x.
Gabler, C.A., M.J. Osland, J.B. Grace, C.L. Stagg, R.H. Day, S.B. Hartley, N.M. Enwright, A.S. From, M.L. McCoy, and J.L. McLeod. 2017. Macroclimatic change expected to transform coastal wetland ecosystems this century. Nature Climate Change 7: 142–147. https://doi.org/10.1038/nclimate3203.
Gabriel, J.R., J. Reid, L. Wang, T.J. Mozdzer, D.F. Whigham, J.P. Megonigal, and J.A. Langley. 2022. Interspecific competition is prevalent and stabilizes plant production in a brackish marsh facing sea level rise. Estuaries and Coasts 45: 1646–1655. https://doi.org/10.1007/s12237-021-01043-9.
Ghannoum, O., S. Von Caemmerer, L.H. Ziska, and J.P. Control. 2000. The growth response of C4 plants to rising atmospheric CO2 partial pressure: A reassessment. Plant, Cell & Environment 23: 931–942. https://doi.org/10.1046/j.1365-3040.2000.00609.x.
Giroux, J.B., and J. Bédard. 1995. Seed production, germination rate, and seedling establishment of Scirpus pungens in tidal brackish marshes. Wetlands 15: 290–297. https://doi.org/10.1007/BF03160709.
HilleRisLambers, J., W.S. Harpole, S. Schnitzer, D. Tilman, and P.B. Reich. 2009. CO2, nitrogen, and diversity differentially affect seed production of prairie plants. Ecology 90: 1810–1820. https://doi.org/10.1890/07-1351.1.
Holler, L.C., and W.G. Abrahamson. 1977. Seed and vegetative reproduction in relation to density in Fragaria virginiana (Rosaceae). American Journal of Botany 64: 1003–1007. https://doi.org/10.1002/j.1537-2197.1977.tb11946.x.
Holmquist, J.R., L. Schile-Beers, K. Buffington, M. Lu, T.J. Mozdzer, J. Riera, D.E. Weller, M. Williams, and J.P. Megonigal. 2021. Scalability and performance tradeoffs in quantifying relationships between elevation and tidal wetland plant communities. Marine Ecology Progress Series 666: 57–72. https://doi.org/10.3354/meps13683.
Humphrey, L.D., and D.A. Pyke. 1998. Demographic and growth responses of a guerrilla and a phalanx perennial grass in competitive mixtures. Journal of Ecology 86: 854–865. https://doi.org/10.1046/j.1365-2745.1998.8650854.x.
Ikegami, M. 2004. Functional spatialization of ramets in a clonal plant network. PhD Thesis. Utrecht University, Utrecht, Nederland.
Ikegami, M., D.F. Whigham, and M.J.A. Werger. 2012. Effects of local density of clonal plants on their sexual and vegetative propagation strategies in a lattice structure model. Ecological Modelling 234: 51–59. https://doi.org/10.1016/j.ecolmodel.2012.03.026.
Kettenring, K.M., D.F. Whigham, E.L.G. Hazelton, S.K. Gallagher, and H.M. Weiner. 2015. Biotic resistance, disturbance, and mode of colonization impact the invasion of a widespread, introduced wetland grass. Ecological Applications 25: 466–480. https://doi.org/10.1890/14-0434.1.
Kettenring, K.M., and D.F. Whigham. 2018. The role of propagule type, resource availability, and seed source in Phragmites invasion in Chesapeake Bay wetlands. Wetlands 38: 1259–1268. https://doi.org/10.1007/s13157-018-1034-5.
Kirwan, M.L., and G.R. Guntenspergen. 2015. Response of plant productivity to experimental flooding in a stable and a submerging marsh. Ecosystems 18: 903–913. https://doi.org/10.1007/s10021-015-9870-0.
Klady, R.A., G.H. Henry, and V. Lemay. 2011. Changes in high arctic tundra plant reproduction in response to long-term experimental warming. Global Change Biology 17: 1611–1624. https://doi.org/10.1111/j.1365-2486.2010.02319.x.
Klimešová, J. 2018. Temperate Herbs: An Architectural Analysis. Praha: Academia.
Koyama, T. 1963. The genus Scirpus Linn.: Critical species of the section Pterolepis. Canadian Journal of Botany 41: 1107–1131. https://doi.org/10.1139/b63-091.
Kudoh, H., and D.F. Whigham. 2001. A genetic analysis of hydrologically dispersed seeds of Hibiscus moscheutos (Malvaceae). American Journal of Botany 88: 588–593. https://doi.org/10.2307/2657057.
Langley, J.A., K.L. McKee, D.R. Cahoon, J.A. Cherry, and J.P. Megonigal. 2009a. Elevated CO2 stimulates marsh elevation gain, counterbalancing sea-level rise. Proceedings of the National Academy of Sciences 106: 6182–6186. https://doi.org/10.1073/pnas.0807695106.
Langley, J.A., and J.P. Megonigal. 2010. Ecosystem response to elevated CO2 levels limited by nitrogen–induced plants species shift. Nature 466: 96–99. https://doi.org/10.1038/nature09176.
Langley, J.A., M.V. Sigrist, J. Duls, D.R. Cahoon, J.C. Lynch, and J.P. Megonigal. 2009b. Global change and marsh elevation dynamics: Experimenting where land meets sea and biology meets geology. Smithsonian Contributions to the Marine Sciences 38: 391–400. https://doi.org/10.5479/si.01960768.38.391.
Langley, J.A., T.J. Mozdzer, K.A. Shepard, S.B. Hagerty, and J.P. Megonigal. 2013. Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Global Change Biology 19: 1495–1503. https://doi.org/10.1111/gcb.12147.
Law, R., A.D. Bradshaw, and P.D. Putwain. 1979. Reply to W. G. Abrahamson. Evolution 33: 519–520. https://doi.org/10.1111/j.1558-5646.1979.tb04706.x.
Lenka, N.K., S. Lenka, J.K. Thakur, R. Elanchezhian, S.B. Aher, V. Simaiya, D.S. Yashona, A.K. Biswas, P.K. Agrawal, and A.K. Patra. 2017. Interactive effect of elevated carbon dioxide and elevated temperature on growth and yield of soybean. Current Science 113: 2305–2310.
Lippmann, R., S. Babben, A. Menger, C. Delker, and M. Quint. 2019. Development of wild and cultivated plants under global warming conditions. Current Biology 29: R1326–R1338. https://doi.org/10.1016/j.cub.2019.10.016.
Loehle, C. 1987. Partitioning of reproductive effort in clonal plants: A benefit–cost model. Oikos 49: 199–208. https://doi.org/10.2307/3566027.
Lu, M., E.R. Herbert, J.A. Langley, M.L. Kirwan, and J.P. Megonigal. 2019. Nitrogen status regulates regular morphological adaptation of marsh plants to elevated CO2. Nature Climate Change 9: 764–768. https://doi.org/10.1038/s41558-019-0582-x.
McCormick, J., and H.A. Jr Somes. 1982. The coastal wetlands of Maryland McCormick J. and Associates Inc Chevy Chase.
McCormick, M.K., K.M. Kettenring, H.M. Baron, and D.F. Whigham. 2010. Extent and reproductive mechanisms of Phragmites australis spread in brackish wetlands in Chesapeake Bay, Maryland (USA). Wetlands 30: 67–74. https://doi.org/10.1007/s13157-009-0007-0.
Messerli, J., A. Bertrand, J. Bourassa, G. Bélanger, Y. Castonguay, G. Tremblay, V. Baron, and P. Sequn. 2015. Performance of low-cost open-top chambers to study long-term effects of carbon dioxide and climate under field conditions. Agronomy Journal 107: 916–920. https://doi.org/10.2134/agronj14.0571.
Miyagi, K.M., T. Kinugasa, K. Hikosaka, and T. Hirose. 2007. Elevated CO2 concentration, nitrogen use, and seed production in annual plants. Global Change Biology 13: 2161–2170. https://doi.org/10.1111/j.1365-2486.2007.01429.x.
Molau, U., and G.R. Shaver. 1997. Controls on seed production and seed germinability in Eriophorum vaginatum. Global Change Biology 3: 80–88. https://doi.org/10.1111/j.1365-2486.1997.gcb130.x.
Mozdzer, T.J., J.A. Langley, P. Mueller, and J.P. Megonigal. 2016. Deep rooting and global change facilitate spread of invasive grass. Biological Invasions 18: 2619–2631. https://doi.org/10.1007/s10530-016-1156-8.
Vanaja, M., M. Maheswari, P. Ratnakumar, and Y.S. Ramakrishna. 2006. Monitoring and controlling CO2 concentrations in open top chambers for better understanding of plants response to elevated CO2 levels. Indian Journal of Radio & Space Physics 35: 193–197.
Nehring, S., and K.J. Hesse. 2008. Invasive alien plants in marine protected areas: The Spartina anglica affair in the European Wadden Sea. Biological Invasions 10: 937–950. https://doi.org/10.1007/s10530-008-9244-z.
Newell, S.J., and E.J. Tramer. 1978. Reproductive strategies in herbaceous plant communities during succession. Ecology 59: 228–234. https://doi.org/10.2307/1936367.
Noyce, G.L., M.L. Kirwan, R.L. Rich, and J.P. Megonigal. 2019. Asynchronous nitrogen supply and demand produce nonlinear plant allocation responses to warming and elevated CO2. Proceedings of the National Academy of Sciences 116: 21623–21628. https://doi.org/10.1073/pnas.1904990116.
Noyce, G.L., A.J. Smith, M.L. Kirwan, R.L. Rich, and J.P. Megonigal. 2023. Oxygen priming induced by elevated CO2 reduces carbon accumulation and methane emissions in coastal wetlands. Nature Geoscience 16: 63–68. https://doi.org/10.1038/s41561-022-01070-6.
Ogden, J. 1974. The reproductive strategy of higher plants: II. The reproductive strategy of Tussilago farfara L. Journal of Ecology 62: 291–324. https://doi.org/10.2307/2258894.
Osanai, Y., D.T. Tissue, M.P. Bange, I.C. Anderson, M.V. Braunack, and B.K. Singh. 2017. Plant-soil interactions and nutrient availability determine the impact of elevated CO2 and temperature on cotton productivity. Plant and Soil 410: 87–102. https://doi.org/10.1007/s11104-016-2981-3.
Pearse, I.S., J.M. LaMontagne, and W.D. Koenig. 2017. Inter-annual variation in seed production has increased over time (1900–2014). Proceedings of the Royal Society B: Biological Sciences 284: 20171666. https://doi.org/10.1098/rspb.2017.1666.
Pastore, M.A., J.P. Megonigal, and J.A. Langley. 2016. Elevated CO2 promotes long–term nitrogen accumulation only in combination with nitrogen addition. Global Change Biology 22: 391–403. https://doi.org/10.1111/gcb.13112.
Pastore, M.A., J.P. Megonigal, and J.A. Langley. 2017. Elevated CO2 and nitrogen addition accelerate net carbon gain in a brackish marsh. Biogeochemistry 133: 73–87. https://doi.org/10.1007/s10533-017-0312-2.
QGIS Development Team. 2002. QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.osgeo.org.
R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Roiloa, S.R., S. Rodriguez-Echeverria, A. Lopez-Otero, R. Retuerto, and H. Freitas. 2014. Adaptive plasticity to heterogeneous environments increases capacity for division of labor in the clonal invader Carpobrotus edulis (Aizoaceae). American Journal of Botany 101: 1301–1308. https://doi.org/10.3732/ajb.1400173.
Smith, S.G. 2002. Schoenoplectus americanus. Flora of North America. vol 23. http://floranorthamerica.org/Schoenoplectus_americanus. Accessed 21 Mar 2023.
Smith, E.P. 2020. Ending reliance on statistical significance will improve environmental inference and communication. Estuaries and Coasts 43: 1–6. https://doi.org/10.1007/s12237-019-00679-y.
Snell, T.W., and D.G. Burch. 1975. The effects of density on resource partitioning in Chamaesyce hirta (Euphorbiaceae). Ecology 56: 742–746. https://doi.org/10.2307/1935512.
Short, F.T., S. Kosten, P.A. Morgan, S. Malone, and G.E. Moore. 2016. Impacts of climate change on submerged and emergent wetland plants. Aquatic Botany 135: 3–17. https://doi.org/10.1016/j.aquabot.2016.06.006.
Solbreck, C., and J. Knape. 2017. Seed production and predation in a changing climate: New roles for resource and seed predator feedback? Ecology 98: 2301–2311. https://doi.org/10.1002/ecy.1941.
Tiner, R.W., and D.G. Burke. 1995. Wetlands of Maryland. Hadley: U.S. Fish and Wildlife Service, Ecological Services and, Annapolis: Maryland Department of Natural Resources, Cooperative publication.
Vahsen, M.L., M.J. Blum, J.P. Megonigal, S.J. Emrich, J.R. Holmquist, B. Stiller, K.E.O. Todd-Brown, and J.S. McLachlan. 2023. Rapid plant trait evolution can alter coastal wetland resilience to sea level rise. Science 379: 393–398. https://doi.org/10.1126/science.abq0595.
Weiss, L., H. Pfestorf, F. May, K. Körner, S. Boch, M. Fischer, J. Müller, D. Prati, S.A. Socher, and F. Jeltsch. 2014. Grazing response patterns indicate isolation of semi-natural European grasslands. Oikos 123: 599–612. https://doi.org/10.1111/j.1600-0706.2013.00957.x.
White, K.P., J.A. Langley, D.R. Cahoon, and J.P. Megonigal. 2012. C3 and C4 biomass allocation responses to elevated CO2 and nitrogen: Contrasting resource capture strategies. Estuaries and Coasts 35: 1028–1035. https://doi.org/10.1007/s12237-012-9500-4.
Williams, R.D., P.C. Quimby, and K.E. Frick. 1977. Intraspecific competition of purple nutsedge (Cyperus rotundus) under greenhouse conditions. Weed Science 25: 477–481. https://doi.org/10.1017/S0043174500033944.
Winn, A.A., and L.F. Pitelka. 1981. Some effects of density on the reproductive patterns and patch dynamics of Aster acuminatus. Bulletin of the Torrey Botanical Club 108: 438–445. https://doi.org/10.2307/2484444.
Zangerl, A.R., and F.A. Bazzaz. 1984. The response of plants to elevated CO2: II. Competitive interactions among annual plants under varying light and nutrients. Oecologia 62: 412–417. https://doi.org/10.1007/BF00384276.
Zedler, J.B., and S. Kercher. 2004. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Critical Reviews in Plant Sciences 23: 431–452. https://doi.org/10.1080/07352680490514673.
Zhu, C., J.A. Langley, L.H. Ziska, D.R. Cahoon, and J.P. Megonigal. 2022. Accelerated sea-level rise is suppressing CO2 stimulation of tidal marsh productivity: a 33-year study. Science Advances 8: eabn0054. https://doi.org/10.1126/sciadv.abn0054.
Ziska, L.H., J.A. Bunce, and F.A. Caulfield. 2001. Rising atmospheric carbon dioxide and seed yield of soybean genotypes. Crop Science 41: 385–391. https://doi.org/10.2135/cropsci2001.412385x.
Acknowledgements
A. K. was supported by Kyoto University OMORO Challenge Scholarship Program. The long-term research at the study sites has been supported by the Smithsonian Institution and the National Science Foundation Long-Term Research in Environmental Biology Program (DEB-0950080, DEB-1457100, DEB-1557009, DEB-2051343), the Department of Energy Terrestrial Ecosystem Science Program (DE-FG02-97ER62458, DE-SC0014413, DE-SC0019110, DE-SC0021112), the United States Geological Survey (G10AC00675), the National Science Foundation Long-Term Research in Environmental Biology Program (DEB-0950080, DEB-1457100, DEB-1557009, DEB-2051343), and the Smithsonian Environmental Research Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Charles T. Roman
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kudoh, A., Megonigal, J.P., Langley, J.A. et al. Reproductive Responses to Increased Shoot Density and Global Change Drivers in a Widespread Clonal Wetland Species, Schoenoplectus americanus. Estuaries and Coasts 47, 176–188 (2024). https://doi.org/10.1007/s12237-023-01249-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01249-z