Abstract
Water column hypoxia, low partial pressure of oxygen (pO2), and hydrogen sulfide (H2S) intrusion, a phytotoxin, are factors linked to global seagrass decline. While many lab experiments have examined these relationships, field studies are needed to elucidate complex drivers of internal pO2 in situ. Herein, we examined plant pO2 and H2S dynamics using microsensors in a dominant tropical seagrass Thalassia testudinum in Florida Bay, a subtropical estuary with recurrent seagrass die-off events. Based on 12 field deployments (48–72 h) across seasons, we show that T. testudinum has a high capacity for daytime leaf oxidation (42–53 kPa) that sustains oxic conditions in its tissues and supersaturates the water column with O2 (> 21 kPa). Although internal daytime O2 is rapidly consumed near sunset, daytime seagrass O2 production leads to supersaturation in the water column beyond sunset. This is an important feedback mechanism as high water column pO2 at night buffers against internal leaf hypoxia via diffusion. Even with high daytime irradiance, however, shoot meristems went anoxic/hypoxic (0.6 kPa) at night, indicating high plant and ecosystem O2 consumption. Hydrogen sulfide was only detected in the meristem when water column pO2 was close to anoxia (< 1 kPa) coincident with maximum water column temperatures (33 °C), an occurrence likely to increase with global warming. Our results support the hypothesis that meristem H2S intrusion in Florida Bay, and likely globally, is primarily driven by insufficient internal plant oxidation by the water column at night, even when high irradiance sustains supersaturation of tissue O2 during the day.








Similar content being viewed by others
References
Armstrong, W. 1979. Aeration in higher plants. In Advances in botanical research, ed. H.W. Woolhouse, 225–332: Academic Press, London.
Barber, T.R., and P.R. Carlson. 1993. Effects of seagrass die-off on benthic fluxes and porewater concentrations of ∑CO2, ∑H2S, and CH4 in Florida Bay sediments. In Biogeochemistry of global change, 530–550.
Beer, S. 1989. Photosynthesis and photorespiration of marine angiosperms. Aquatic Botany 34: 153–166.
Beer, S., M. Björk, F. Hellblom, and L. Axelsson. 2002. Inorganic carbon utilization in marine angiosperms (seagrasses). Functional Plant Biology 29: 1–6.
Binzer, T., J. Borum, and O. Pedersen. 2005. Flow velocity affects internal oxygen conditions in the seagrass Cymodocea nodosa. Aquatic Botany 83: 239–247.
Borum, J., O. Pedersen, T.M. Greve, T.A. Frankovich, J.C. Zieman, J.W. Fourqurean, and C.J. Madden. 2005. The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. Journal of Ecology 93: 148–158.
Borum, J., K. Sand-Jensen, T. Binzer, O. Pedersen, and T.M. Greve. 2006. Oxygen movement in seagrasses. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, R.J. Orth and C. Duarte, 255–270. Dordrecht: Springer Netherlands.
Brodersen, K.E., K.J. Hammer, V. Schrameyer, A. Floytrup, M.A. Rasheed, P.J. Ralph, M. Kühl, and O. Pedersen. 2017. Sediment resuspension and deposition on seagrass leaves impedes internal plant aeration and promotes phytotoxic H2S intrusion. Frontiers in Plant Science 8: 1–13.
Brodersen, K.E., K. Koren, M. Lichtenberg, and M. Kühl. 2016. Nanoparticle-based measurements of pH and O2 dynamics in the rhizosphere of Zostera marina L.: Effects of temperature elevation and light-dark transitions. Plant Cell and Environment 39: 1619–1630.
Brodersen, K.E., M. Kühl, D.A. Nielsen, O. Pedersen, and A.W.D. Larkum. 2018a. Rhizome, root/sediment interactions, aerenchyma and internal pressure changes in seagrasses. Seagrasses of Australia: Structure, Ecology and Conservation: 393–418.
Brodersen, K.E., M. Lichtenberg, L.-C. Paz, and M. Kühl. 2015a. Epiphyte-cover on seagrass (Zostera marina L.) leaves impedes plant performance and radial O2 loss from the below-ground tissue. Frontiers in Marine Science 2: 1–11.
Brodersen, K.E., D.A. Nielsen, P.J. Ralph, and M. Kühl. 2014. A split flow chamber with artificial sediment to examine the below-ground microenvironment of aquatic macrophytes. Marine Biology 161: 2921–2930.
Brodersen, K.E., D.A. Nielsen, P.J. Ralph, and M. Kühl. 2015b. Oxic microshield and local pH enhancement protects Zostera muelleri from sediment derived hydrogen sulphide. New Phytologist 205: 1264–1276.
Brodersen, K.E., N. Siboni, D.A. Nielsen, M. Pernice, P.J. Ralph, J. Seymour, and M. Kühl. 2018b. Seagrass rhizosphere microenvironment alters plant-associated microbial community composition. Environmental Microbiology 20: 2854–2864.
Buapet, P., L.M. Rasmusson, M. Gullstrom, and M. Bjork. 2013. Photorespiration and carbon limitation determine productivity in temperate seagrasses. PLoS One 8: e83804.
Burkholder, J.M., D.A. Tomasko, and B.W. Touchette. 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology 350: 46–72.
Calleja, M.L., C. Barrón, J.A. Hale, T.K. Frazer, and C.M. Duarte. 2006. Light regulation of benthic sulfate reduction rates mediated by seagrass (Thalassia testudinum) metabolism. Estuaries and Coasts 29: 1255–1264.
Carlson, P.R., L.A. Yarbro, and T.R. Barber. 1994. Relationship of sediment sulfide to mortality of Thalassia testudinum in Florida Bay. Bulletin of Marine Science 54: 733–746.
Chambers, R.M., J.W. Fourqurean, S.A. Macko, and R. Hoppenot. 2001. Biogeochemical effects of iron availability on primary producers in a shallow marine carbonate environment. Limnology and Oceanography 46: 1278–1286.
Cline, J.D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnology and Oceanography 14: 454–458.
Colmer, T.D. 2003. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 26: 17–36.
Duarte, C.M. 1991. Seagrass depth limits. Aquatic Botany 40: 363–377.
Fredley, J., M.J. Durako, M.O. Hall, and F. Bay. 2019. Multivariate analyses link macrophyte and water quality indicators to seagrass die-off in Florida Bay. Ecological Indicators 101: 692–701.
Greve, T.M., J. Borum, and O. Pedersen. 2003. Meristematic oxygen variability in eelgrass (Zostera marina). Limnology and Oceanography 48: 210–216.
Greve, T.M., and D. Krause-Jensen. 2005. Predictive modelling of eelgrass (Zostera marina) depth limits. Marine Biology 146: 849–858.
Hall, M.O., B.T. Furman, M. Merello, and M.J. Durako. 2016. Recurrence of Thalassia testudinum seagrass die-off in Florida Bay, USA: Initial observations. Marine Ecology Progress Series 560: 243–249.
Han, C., J.H. Ren, P.N. Williams, F. Ke, Q.S. Shen, Z.D. Wang, D. Xu, and J. Luo. 2019. High-resolution imaging of rhizosphere oxygen (O2) dynamics in Potamogeton crispus: Effects of light, temperature and O2 content in overlying water. Plant and Soil 441: 613–627.
Holmer, M. 2003. Sulfur cycling and seagrass (Posidonia oceanica) status in carbonate sediments. Biogeochemistry 66: 223–239.
Holmer, M., and H. Hasler-Sheetal. 2014. Sulfide intrusion in seagrasses assessed by stable sulfur isotopes–A synthesis of current results. Frontiers in Marine Science 1: 1–12.
Holmer, M., O. Pedersen, D. Krause-Jensen, B. Olesen, M. Hedegård Petersen, S. Schopmeyer, M. Koch, B.A. Lomstein, and H.S. Jensen. 2009. Sulfide intrusion in the tropical seagrasses Thalassia testudinum and Syringodium filiforme. Estuarine, Coastal and Shelf Science 85: 319–326.
Johnson, C.R., M.S. Koch, O. Pedersen, and C.J. Madden. 2018. Hypersalinity as a trigger of seagrass Thalassia testudinum die-off events in Florida Bay: Evidence based on shoot meristem O2 and H2S dynamics. Journal of Experimental Marine Biology and Ecology 504: 47–52.
Johnson, C.R., M.S. Koch, O. Pedersen, and C.J. Madden. 2020. Hypersalinity affects leaf and meristem O2 dynamics exposing meristems to H2S in the dominant tropical seagrass Thalassia testudinum. Journal of Experimental Marine Biology and Ecology 533: 1–10.
Koch, M., G. Bowes, C. Ross, and X.H. Zhang. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology 19: 1–29.
Koch, M.S., and J.M. Erskine. 2001. Sulfide as a phytotoxin to the tropical seagrass Thalassia testudinum: Interactions with light, salinity and temperature. Journal of Experimental Marine Biology and Ecology 266: 81–95.
Koch, M.S., S. Schopmeyer, C. Kyhn-Hansen, and C.J. Madden. 2007a. Synergistic effects of high temperature and sulfide on tropical seagrass. Journal of Experimental Marine Biology and Ecology 341: 91–101.
Koch, M.S., S.A. Schopmeyer, M. Holmer, C.J. Madden, and C. Kyhn-Hansen. 2007b. Thalassia testudinum response to the interactive stressors hypersalinity, sulfide and hypoxia. Aquatic Botany 87: 104–110.
Koch, M.S., S.A. Schopmeyer, O.I. Nielsen, C. Kyhn-Hansen, and C.J. Madden. 2007c. Conceptual model of seagrass die-off in Florida Bay: Links to biogeochemical processes. Journal of Experimental Marine Biology and Ecology 350: 73–88.
Koren, K., K.E. Brodersen, S.L. Jakobsen, and M. Kühl. 2015. Optical sensor nanoparticles in artificial sediments-A new tool to visualize O2 dynamics around the rhizome and roots of seagrasses. Environmental Science and Technology 49: 2286–2292.
Ku, T.C.W., L.M. Walter, M.L. Coleman, R.E. Blake, and A.M. Martini. 1999. Coupling between sulfur recycling and syndepositional carbonate dissolution: Evidence from oxygen and sulfur isotope composition of pore water sulfate, South Florida Platform, U.S.A. Geochimica Et Cosmochimica Acta 63: 2529–2546.
Lamers, L.P., L.L. Govers, I.C. Janssen, J.J. Geurts, M.E. Van der Welle, M.M. Van Katwijk, T. Van der Heide, J.G. Roelofs, and A.J. Smolders. 2013. Sulfide as a soil phytotoxin-A review. Frontiers in Plant Science 4: 268.
Larkum, A.W.D., E.A. Drew, and P.J. Ralph. 2006. Photosynthesis and metabolism in seagrasses at the cellular level. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, 323–345. Netherlands: Springer.
Larkum, A.W.D., A.J. McComb, and S.A. Sheperd. 1989. Gaseous movement in seagrasses. In Biology of seagrasses, ed. A.W.D. Larkum, A.J. McComb and S.S. A., 686–722. The Netherlands: Elsevier.
Lee, K.S., and K.H. Dunton. 2000. Diurnal changes in pore water sulfide concentrations in the seagrass Thalassia testudinum beds: The effects of seagrasses on sulfide dynamics. Journal of Experimental Marine Biology and Ecology 255: 201–214.
Martin, B.C., J. Bougoure, M.H. Ryan, W.W. Bennett, T.D. Colmer, N.K. Joyce, Y.S. Olsen, and G.A. Kendrick. 2019. Oxygen loss from seagrass roots coincides with colonisation of sulphide-oxidising cable bacteria and reduces sulphide stress. ISME Journal 13: 707–719.
Millero, F.J. 1986. The thermodynamics and kinetics of the hydrogen sulfide in natural waters. In Marine Chemistry, 121–147.
Nguyen, H.M., P.J. Ralph, L. Marin-Guirao, M. Pernice, and G. Procaccini. 2021. Seagrasses in an era of ocean warming: a review. Biological Reviews of the Cambridge Philosophical Society 1–22.
Olsen, Y.S., M.W. Fraser, B.C. Martin, A. Pomeroy, R. Lowe, O. Pedersen, and G.A. Kendrick. 2018. In situ oxygen dynamics in rhizomes of the seagrass Posidonia sinuosa: Impact of light, water column oxygen, current speed and wave velocity. Marine Ecology Progress Series 590: 67–77.
Oremland, R.S., and B.F. Taylor. 1977. Diurnal fluctuations of O2, N2, and CH4 in the rhizosphere of Thalassia testudinum. Limnology and Oceanography 22: 566–570.
Orth, R.J., T.J.B. Carruthers, W.C. Dennison, C.M. Duarte, J.W. Fourqurean, K.L. Heck, A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, S. Olyarnik, F.T. Short, M. Waycott, and S.L. Williams. 2006. A global crisis for seagrass ecosystems. BioScience 56: 987–996.
Pedersen, O., T. Binzer, and J. Borum. 2004. Sulphide intrusion in eelgrass Zostera marina L. Plant, Cell & Environment 27: 595–602.
Pedersen, O., J. Borum, C. Duarte, and M. Fortes. 1998. Oxygen dynamics in the rhizosphere of Cymodocea rotundata. Marine Ecology Progress Series 169: 283–288.
Pedersen, O., T.D. Colmer, J. Borum, A. Zavala-Perez, and G.A. Kendrick. 2016. Heat stress of two tropical seagrass species during low tides - Impact on underwater net photosynthesis, dark respiration and diel in situ internal aeration. New Phytologist 210: 1207–1218.
Pedersen, O., N.P. Revsbech, and S. Shabala. 2020. Microsensors in plant biology: In vivo visualization of inorganic analytes with high spatial and/or temporal resolution. Journal of Experimental Botany 71: 3941–3954.
Raun, A.L., and J. Borum. 2013. Combined impact of water column oxygen and temperature on internal oxygen status and growth of Zostera marina seedlings and adult shoots. Journal of Experimental Marine Biology and Ecology 441: 16–22.
Raven, J.A., and C.M. Scrimgeour. 1997. The influence of anoxia on plants of saline habitats with special reference to the sulphur cycle. Annals of Botany 79: 79–86.
Robblee, M.B., T.R. Barber, P.R. Carlson, M.J. Durako, J.W. Fourqurean, L.K. Muehlstein, D. Porter, L.A. Yarbro, R.T. Zieman, and J.C. Zieman. 1991. Mass mortality of the tropical seagrass Thalassia testudinum in Florida Bay (USA). Marine Ecology Progress Series 71: 297–299.
Rosch, K.L., and M.S. Koch. 2009. Nitrogen and phosphorus recycling by a dominant tropical seagrass Thalassia testudinum across a nutrient gradient in Florida Bay. Bulletin of Marine Science 84: 1–24.
Sand-Jensen, K., O. Pedersen, T. Binzer, and J. Borum. 2005. Contrasting oxygen dynamics in the freshwater isoetid Lobelia dortmanna and the marine seagrass Zostera marina. Annals of Botany 96: 613–623.
Seddon, S., R.M. Connolly, and K.S. Edyvane. 2000. Large-scale seagrass dieback in northern Spencer Gulf, South Australia. Aquatic Botany 66: 297–310.
Smith, R.D., W.C. Dennison, and R.S. Alberte. 1984. Role of seagrass photosynthesis in root aerobic processes. Plant Physiology 74: 1055–1058.
Timbs, R., and M.J. Durako. 2021. Landscape-scale variation in a sulfur-based sediment stress indicator for the seagrass Thalassia testudinum in Florida Bay, USA. Marine Ecology Progress Series 670: 33–47.
Waycott, M., C.M. Duarte, T.J.B. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnik, A. Calladine, J.W. Fourqurean, K.L. Heck, A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, F.T. Short, and S.L. Williams. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106: 12377–12381.
Zhang, J.-Z., C.J. Fischer, and P.B. Ortner. 2004. Potential availability of sedimentary phosphorus to sediment resuspension in Florida Bay. Global Biogeochemical Cycles 18: 1–14.
Zieman, J.C., J.W. Fourqurean, and T.A. Frankovich. 1999. Seagrass die-off in Florida Bay: Long-term trends in abundance and growth of turtle grass, Thalassia testudinum. Estuaries 22: 460–470.
Zieman, J.C., J.W. Fourqurean, and R.L. Iverson. 1989. Distribution, abundance and productivity of seagrasses and macroalgae in Florida Bay. Bulletin of Marine Science 44: 292–311.
Zimmerman, R.C. 2020. Scaling up: Predicting the impacts of climate change on seagrass ecosystems. Estuaries and Coasts 44: 558–576.
Acknowledgements
We thank Zachary W. Fratto from Everglades National Park (ENP) for providing the ENP monitoring data from our site, Kasey McLeod for her support working in the field, and Unisense Inc. for their technical expertise and troubleshooting of microsensors. Two anonymous reviewers are thanked for improving the manuscript.
Funding
This work was supported by the South Florida Water Management District (#4500113844).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Melisa C. Wong.
Supplementary Information
Below is the link to the electronic supplementary material.
12237_2022_1064_MOESM1_ESM.docx
Supplementary file1: Table S1. The beginning and end dates of each microsensor field deployment and their ID numbers given in supplemental tables in the manuscript. The number of day/night replicates during each deployment are presented in parentheses.
Table S2. Seasonal daytime (sunrise-sunset) water column physiochemical parameters and irradiance (I) during 29 individual days of seasonal deployments within Thalassia testudinum meadows at the Johnson Key study site in Florida Bay. Average, minimum, and maximum daytime water column pO2 (kPa), temperature (oC), and salinity, and average and maximum canopy height irradiance (µmol photons m-2 s-1) and day length (DL) are presented. Data was collected every minute during deployments. Parameters were averaged by season (a) winter, (b) spring, (c) summer, (d) fall. Significant differences among season are denoted by superscript letters (ANOVA, post-hoc Tukey, p < 0.05). Parameter variance, minimum and maximum ranges for each date and the beginning and ending times of each record are presented. Deployment # in parentheses (Table S1). na = data not available.
Table S3. Nighttime seasonal water column physiochemical parameters and irradiance during 20 individual nights during seasonal deployments within Thalassia testudinum meadows at the Johnson Key study site in Florida Bay. Average, minimum and maximum nighttime water column pO2, temperature, and salinity are presented. Data was collected every minute during deployments. Significant differences among season denoted by superscript letters (P < 0.01 temperature; P < 0.05 salinity; P = 0.07 pO2 max). Parameter variance for each date and the beginning and ending times of each record are presented. Deployment # in parentheses (Table S1). na = data not available.
Table S4. Seasonal porewater (10-15 cm depth) salinity, pHpw, and total sulfide (ΣTSpw). Gaseous H2S was calculated based on total sulfide, temperature, salinity, and pH according to Millero (1986). Parameters were averaged by season (a) winter, (b) spring, (c) summer, (d) fall. Seasonal significance (p < 0.05) is denoted by superscript letters (ANOVA, post-hoc Tukey), Means ± SD (n = 5).
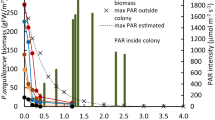
Fig. S1. The sequence of cues for meristem placement is described below and illustrated in this schematic of a meristem cross-section of Thalassia testudium from the Johnson Key site in Florida Bay. We observed a sequence of Δ pO2 as the microsensors were moved into the shoot meristem as follows: (1) the ambient water column pO2 is measured as the O2 sensor approached the meristem, (2) a rapid drop in pO2 was detected at the surface of the non-photosynthetic base of the outer leaf sheath, (3) an increase in pO2 was detected once inside the aerenchyma tissue of the outermost leaf sheath, (4) a drop in pO2 occurred as the sensor penetrated the inner leaf sheath, and (5) a pO2 above the water column was detected once inside the meristem tissue aerenchyma of the inner leaf sheath. Location of sensor placement in the meristem is shown in Fig. 2c.
Fig. S2. Linear (right column) and non-linear or bi-phasic (left column) leaf pO2 dynamics from sunrise to 11:00 am during full sun deployments (n = 8) across seasons: (a) November 7, 2018 (b) February 17, 2019, (c) November 19, 2019, (d) March 9th, 2019, (e) April 18, 2019 (f) June 22, 2019, (g) September 19, 2019, (h) November 20, 2019. See Table 2 for regression details.
Fig. S3. Leaf pO2 from maximum in the afternoon to sunset. Irradiance during this time is also shown and the lag of leaf pO2 decline in response to decreasing irradiance identified with a double arrow. The lag times and leaf Δ pO2 following the lag are presented in Table 4. Replicates include those with afternoon leaf pO2 dynamics through sunset (n = 10).
Fig. S4. Linear relationship between leaf and meristem partial pressure during the morning (sunrise to 11:00) for all seasons. The date on the figures is the day microsensor measurements were taken.
Fig. S5. Linear relationship between leaf and meristem partial pressure during the afternoon (14:00 to sunset) for all seasons. The date on the figures is the day microsensor measurements were taken.
Fig. S6. Rates of decline in leaf (dark line) and meristem (grey line) pO2 following sunset. Linear regressions and equations are shown on graphs from sunset to when internal pO2 reached hypoxia (1.5 kPa) or sunrise the following day. Further data on nighttime parameters are presented in Table 6.
Fig. S7. Nighttime leaf and meristem pO2 as a function of water column pO2 during replicates where both leaf and meristem microsensor data were available. Dates of replicates on shown on each graph. Linear regression lines, linear equations, and unity lines (dashed) are shown. Arrows indicate area of line where regression was determined. Regression information is presented in Table 7within manuscript.
Fig. S8. Nighttime leaf and meristem pO2 as a function of water column pO2 during each replicate where only leaf or meristem microsensor data were available. Leaf data are shown in black and in the two right panels, while the meristem data are in grey and on the left three panels. Dates of replicates are shown on each graph. Linear regression lines, linear equations, and unity lines (dashed) are shown. Regression information is presented in Table 7.
Fig. S9. Temperature (top) and dissolved oxygen (bottom) in 30 min intervals from May 1 to October 1, 2019 in the water column at the long-term hydrostation of Everglades National Park adjacent to Johnson Key (JK) within about 200 meters of our microsensor measurements within Thalassia testudinum meadows. Arrows depict the June 21-22, 2019 date where meadow surface waters, leaves and meristems were hypoxic and H2S intrusion was detected in the meristem. Note: the ENP JK water quality station does not support underlying dense seagrass and is close to the mangrove fringe of JK; thus, the absolute water quality values may differ from those measured within the seagrass canopy reported herein. (DOCX 4724 KB)
Rights and permissions
About this article
Cite this article
Koch, M.S., Johnson, C.R., Madden, C.J. et al. Irradiance, Water Column O2, and Tide Drive Internal O2 Dynamics and Meristem H2S Detection in the Dominant Caribbean-Tropical Atlantic Seagrass, Thalassia testudinum. Estuaries and Coasts 45, 2543–2559 (2022). https://doi.org/10.1007/s12237-022-01064-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01064-y