Abstract
The capture of incoming solar radiation under unlimited light, water, and nutrient conditions by plant canopies and converting it into biomass has been described as radiation use efficiency (RUE). RUE has been computed as a function of biomass accumulation and intercepted photosynthetically active radiation without considering the loss of photoassimilates due to respiratory processes. This study evaluated the RUE in diploid potato crop (Solanum phureja Juz. et Buk.) across six experiments in Colombia. Total biomass was measured during the crop season from the early vegetative stage through maturity. However, this proposal involves not only the total biomass accumulated concerning the amount of photosynthetically active radiation intercepted but also took into account the losses by respiration, following Thornley respiration approach. This research demonstrates that the RUE is not a constant value as the respiration process leads to RUE values being variable in a non-linear way over time. The daily RUE simulation, conducted through an interpolation process, revealed significant variation from emergence to the end of the cycle. This indicates an error in assuming a constant RUE throughout the entire growth period, particularly in assessing its physiological impact across the entire growth and development crop cycle.
Resumen
La captura de la radiación solar incidente por el follaje de las plantas y su conversión en biomasa en condiciones ilimitadas de luz, agua y nutrientes se ha descrito como eficiencia en el uso de la radiación (RUE). El valor de RUE se ha calculado en función de la acumulación de biomasa y de la radiación fotosintéticamente activa interceptada sin considerar la pérdida de fotoasimilados debido a procesos respiratorios. Este estudio evaluó el RUE en el cultivo de papa diploide (Solanum phureja Juz. et Buk.) a través de seis experimentos en Colombia. La biomasa total se midió durante el ciclo de cultivo, desde la etapa vegetativa temprana hasta la madurez. Sin embargo, esta propuesta no solo involucra a la biomasa total acumulada en relación con la cantidad de radiación fotosintéticamente activa interceptada, sino que también tuvo en cuenta las pérdidas por respiración, siguiendo el modelo de respiración de Thornley. Esta investigación demuestra que el RUE no es un valor constante, ya que el proceso de respiración hace que los valores de RUE sean variables de forma no lineal a lo largo del tiempo. La simulación diaria de RUE, realizada a través de un proceso de interpolación, reveló una variación significativa desde la emergencia hasta el final del ciclo. Esto sugiere un error al suponer un RUE constante a lo largo de todo el período de crecimiento, particularmente en la evaluación de su impacto fisiológico a lo largo de todo el ciclo de cultivo.
Similar content being viewed by others
Introduction
Crop growth and yield are mainly determined by four key processes: a) the interception of incident photosynthetically active radiation, b) the utilization of solar energy in reducing substrates (CO2) during photosynthesis, c) the integration of photoassimilates into new plant structures, and finally, d) the maintenance of these plant structures (Loomis and Amthor 1999; Zhu et al. 2008). Plant growth involves intricate processes, such as the dynamic partitioning of biomass, root, and foliage development, typically established prior to the onset of reproductive efforts. Additionally, the cost of maintenance rises as biomass accumulates during the crop cycle, alongside the cost of assimilate synthesis (Weraduwage et al. 2015).
Yield and crop growth are attributed to photosynthesis but, the correlation between net photosynthesis and crop productivity lacks robustness due to leaf display and shading within the canopy (Bassham and Lambers 2021; Niinemets 2010). In the early stages of development, leaves are fully exposed to incident solar radiation, allowing for maximum photosynthesis rates. However, as the crop progresses through vegetative and reproductive growth, only a few leaves remain entirely exposed (Dewar 1996). Other factors contributing to the weak correlation may include adaptations to radiation, temperature, environmental stress, planting density, and the subsequent increase in maintenance respiration (Loomis and Amthor 1999; Loomis and Williams 1963).
In order to establish an appropriate association between the increase in dry matter and the process of photosynthesis, significant attention has been paid to the light conversion efficiency, usually referred to as radiation use efficiency (RUE). RUE has been measured as the relationship between the total biomass produced and solar radiation intercepted by the canopy (g MJ−1) (Murchie et al. 2019). The optimal RUE would be reached with all leaves exposed to an adequate solar radiation amount with no light saturation. If the crop simultaneously intercepted most of the incoming radiation, the dry matter production rate would also be upgraded (Stöckle et al. 2008).
De Wit (1959) and Loomis and Williams (1963) stand out as pioneering authors who conducted insightful analyses on the correlation between biomass increments and the amount of radiation accessible to a crop. De Wit (1959) considered a constant radiation use efficiency at the leaf level up to a saturating light value and examined the consequences of light distribution in a leaf canopy, and the canopy photosynthetic ability was determined to be proportional to the solar radiation incident to the canopy.
Loomis and Williams (1963) advanced this standby considering the quantum nature of radiation and expressing the efficiency of using radiation in terms of accumulated biomass. In this approximation, assuming a maximum value for leaf quantum efficiency of 10 quanta per CO2 fixed, the authors computed that the limit for crop efficiency was 3.35 g of dry matter per MJ−1.
Monteith (1963) distinguished the plant function in intercepting and absorbing solar radiation from its purpose in transforming intercepted solar radiation into biomass. Then, the relationship between growth and intercepted radiation was characterized by Warren Wilson (1967). The author declared a linear relationship between biomass accumulation and the time integral of radiation interception by the plants.
The burden in correlating productivity with incoming solar radiation lies in the fact that plants intercept only a fraction of the incident radiation throughout their life cycle, making it accessible for photosynthesis. Disparities in canopy development across various crops, locations, and climates can complicate the comparison of efficiency solely relying on incident light (Squire 1990).
Monteith (1972) remarked that classifying ecosystems based on efficiency concerning incident radiation is becoming a widespread form of taxonomy, but it contributes slightly to our understanding of how plants respond to the environment. Monteith (1972) moved forward, relating efficiencies of dry matter production to the physical and biological factors that determine growth rates such as the fraction of radiation intercepted by the canopy, the irradiance of individual leaves, the diffusion resistance of stomata, and the behavior of the photochemical system.
Loomis and Amthor (1999) proposed that the term 'radiation use efficiency' (RUE) is suitable for establishing a meaningful connection between yield and net photosynthesis. Although terms such as ‘radiation conversion factor’ and ‘radiation conversion efficiency’ have been used, it has been denoted that the use of the word ‘conversion’ is inappropriate because energy is not being directly converted to biomass but instead converted from one form of energy to another during photosynthesis reactions (Sinclair and Muchow 1999). Moreover, the concept of RUE has also been widely used as an important method of modeling and analyzing crop growth and development (Bonhomme 2000). And most programs or crop simulation models consider two or three values of RUE during the development of the crop (Brown et al. 2011; Griffin et al. 1993; Haverkort et al. 2015; Hoogenboom et al. 2018; Ritchie et al. 1995; Singh et al. 2005).
Estimation of RUE from Biomass Production and Radiation Interception Measurements
The RUE is generally considered as a constant and is computed as the slope of the relation between total biomass (g m−2) and cumulative intercepted radiation (MJ m−2) (Murchie et al. 2019; Stöckle and Kemanian 2009). In a comparative study among various species, Murata (1981) observed that C4 plants, characterized by higher leaf photosynthetic rates, yielded greater RUE values (2.48 g MJ−1) compared to C3 plants (1.82 g MJ−1), as determined by the usual method of RUE estimation. Since the research carried out by Sale (1974), there have been reports on the relationship between radiation interception and dry matter accumulation in potato crops. However, a noteworthy variation in RUE values, ranging from 1.3 to 4.3 g MJ−1, has been observed (Table 1). This variation may, in part, stem from differences in the methodologies employed for measuring PAR and its interception, estimating LAI, the influence of environmental factors on regular crop development and growth, and variations among different cultivars. A detailed review of factors that contribute to uncertainty and errors in RUE determination is given by Sinclair and Muchow (1999) and Gallo et al. (1993).
Some Sources of Variation in Agricultural RUE
The value of the RUE is sensitive to factors affecting the crop, e.g., the amount of incident and intercepted radiation, the variation in maintenance respiration, which depends on temperature (Tjoelker et al. 2001), and the photoassimilates used per gram new biomass increases (growth respiration) (Thornley 1977). Therefore, the actual RUE occurs for plants with a large carbohydrates content (starch, sugars, cellulose) under ideal environmental conditions (Loomis and Amthor 1999).
Crop, weather, and soil variability are important sources of uncertainty in RUE calculation from total dry matter and the intercepted PAR and also requires sufficient samples to measure biomass with confidence. The moments at which measurements are made are critical due to RUE varies according to crop developmental state (Murchie et al. 2019). Furthermore, the measurement of radiation interception should be done continuously throughout the growing season, which is logistically problematic on many occasions (Campbell and Norman 2011; Stöckle and Kemanian 2009). The field method has to consider the frequency of measurements, time of day, and sensor placement to provide correct daily integration of radiation interception (Jing et al. 2012).
The crop development state affects the RUE value achieved because the capacity to intercept the incident radiation, and the potential photosynthetic rate depends on the stage of growth (Murchie et al. 2019; Trapani et al. 1992). Furthermore, the appearance and disappearance of vegetative and reproductive organs can influence photosynthesis through the signaling mechanisms of accumulating carbohydrates. In some species, a higher RUE was observed during the early stages of crop establishment than later stages, which was attributed to a higher photosynthetic capacity (Sinclair and Muchow 1999). Even with this evidence, in practice, it is calculated as a constant which is only modified in two or three stages of crop cycle; it is not considered that the RUE can change continuously.
Another environmental factor that affects the RUE is the nitrogen (N) concentration. It is expected that N has an impact on RUE because of the well-established relationship between the leaf photosynthesis and Rubisco content with the N concentration (Evans 1989; Zhou et al. 2017). Large concentrations of N are required for the building of dense canopies during the vegetative growth; under N deficient conditions, the plant compensates for the lack of N by producing a lower number of leaves and smaller leaves, affecting the radiation interception and crop photosynthesis (Loomis and Gerakis 1975). This, in turn, results in less intercepted radiation and, thereby, less RUE.
The temperature should be associated with the RUE because it influences all vital processes of the plant. The temperature regulates the reactions involved in the processes, starting with the rate of assimilation of CO2 in the leaves, the synthesis of sugars for immediate use, the synthesis of starches for storage, and the respiratory processes of maintenance of existing structures (Sinclair and Muchow 1999; Thornley 2011). Plant biology researchers have long used Q10 to describe the temperature dependence of rates of respiration processes. The Q10 function assumes an exponential relationship with temperature in which Q10 is the ratio of the respiration rate at one temperature to that at a temperature 10 °C lower (Tjoelker et al. 2001). A fixed Q10 of 2.0 has gained wide acceptance in modeling leaf to ecosystem-scale respiration responses to temperature (Smith and Dukes 2013). Maintenance respiration also increases during the crop senescence, and consequently, lower RUE values would be expected during the senescence than in early and reproductive stages (Loomis and Amthor 1999; McCree 1982).
As a generalization, it is acceptable to state that any factor, biotic or abiotic, that reduces the canopy photosynthetic potential and increases the respiration process and is also likely to reduce the RUE. Nevertheless, this statement applies to many common factors that reduce potential growth, such as water availability, nutrients, diseases, pests, and agronomic management. In this sense, increasing the capability of leaves to intercept the incoming radiation directly relates to increased RUE. According to the RUE characteristics and its importance in understanding crop productivity, this study aimed to estimate the RUE of the diploid potato crop as a case study. This assessment involved not only the total biomass accumulated concerning the amount of PAR intercepted but also considered the losses by respiration. In this way, estimation of the RUE is not constant throughout the crop, and this research presents an adequate solution to this problem. This approach can also be applied to any crop.
Materials and Methods
Location and Plant Material
We conducted a case study in the diploid potato crop to illustrate the methods for estimating Radiation Use Efficiency (RUE) while accounting for respiration losses. Six experiments were employed to assess RUE using the diploid potato cultivar ‘Colombia’. Three trials were conducted consecutively, while the other three occurred simultaneously in three locations across Colombia. The data for this research was sourced from Saldaña-Villota and Cotes-Torres (2020), and Patiño (2014). Fertilizer management, aligned with the soil conditions and the nutritional requirements of the diploid potato crop (Guerrero 1998), ensured that the demand for N, P, and K was met, and nutritional factors were not limiting. Detailed information about the experiments can be found in Table 2.
Experimental Design
A randomized block design with five replications was employed for each growing season. Whole plants within a one-meter square area of each experimental unit were harvested on a weekly basis. The criterion for selecting harvested plants for biomass determination was that they had to be entirely surrounded by neighboring plants, experiencing complete crop competition. Consequently, no samples were taken from the borders of the plots. The experimental design ensured an ample number of plants per plot to account for samples from vacant spaces. The variables assessed included total leaf area, measured using a LI-COR leaf area meter (Model 3100, LI-COR Biosciences), and total dry matter. During harvesting, each plant underwent dissection for dry matter measurement, with each organ separated and packed into paper bags. The seed piece was excluded from the measurements of plant mass. The plants were dried at 80 ºC until a constant dry weight was achieved. Subsequently, the total dry weight was determined by summing the dry weights of all plant components.
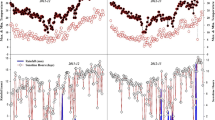
Daily weather data were recorded with a WatchDog 2900ET station for each site and included total solar radiation, precipitation, and maximum and minimum temperature. Missing data of daily precipitation were replaced with weather data obtained from Sistema de Alerta Temprana de Medellín y del Valle de Aburrá (SIATA) and from Instituto de Hidrología, Meteorología y Estudios Ambientales—Ministerio de Ambiente y Desarrollo Sostenible de Colombia (IDEAM) (Fig. 1).
Daily weather data. Daily solar radiation (orange line) (left ordinate axis) (MJ m−2) and daily precipitation (blue bars) (right ordinate axis) (mm day−1) during the six crop growth seasons (abscissa axis) (month/day/year) at three locations in Colombia. Figures a, b, c, and d figures correspond to the field experiments established in Medellín, Colombia. Figure e corresponds to the field experiment established in La Unión, Colombia. And figure f corresponds to the field experiment established in San Pedro de los Milagros, Colombia. The planting and the harvest date of each experiment is represented by the black dotted lines
According to McCree (1971), photosynthetically active radiation (PAR) corresponds to 44% of the incident solar radiation. Thus,
Where \({p}_{t}\) is the photosynthetically active radiation (PAR, usually measured in MJ m−2), and \({I}_{t}\) is the incoming solar radiation (usually measured in MJ m−2).
The fractional PAR intercepted by the canopy (\({i}_{t}\)) was obtained following the Eq. (2) according to the analogy with Beer–Lambert law that states the attenuation of radiation in a homogeneous medium depends on the incident irradiation, the canopy architecture, and the optical properties (Monsi and Saeki 1953):
where LAI is the leaf area index, and k corresponds to the radiation extinction coefficient, which changes with leaf age.
Estimation of RUE without considering the respiration processes
Many studies, such as those mentioned in Table 1, consider estimating a constant RUE as the slope of the relationship between the total biomass (wt) and the intercepted radiation (Murchie et al. 2019; Stöckle and Kemanian 2009). The approach in mathematical terms is the following:
where λ is the constant RUE for the entire growth period without considering the respiration.
where T is the growth time considered for the estimation.
Define \({A}_{t}={\int }_{0}^{T}{p}_{t}{i}_{t}{d}_{t}\) which is the PAR absorbed by the crop up to time t, and \({W}_{t}={\int }_{0}^{T}{w}_{t}{d}_{t}\) which is the cumulated biomass up to time t, then
thus, a linear regression obtained between \({W}_{t}\) and \({A}_{t}\) would allow us to know λ (RUE) that clearly does not consider the respiration processes.
Estimation of RUE considering the respiration processes
This section explores how to involve respiration in estimating RUE both in a general way and using a classical respiration model proposed by Thornley (1970) (Thornley 1970) under controlled and field conditions.
Estimation of a constant RUE considering the respiration processes
Starting from the total cumulated dry matter and from Eq. (7), we obtain:
where \({\Lambda }_{t}\) is the actual RUE in the time t and \({r}_{t}\) is the respiration (g) in time t.
Relationship Between RUE with and Without Considering the Respiration Processes
To see if the RUE obtained without considering respiration has a linear relationship with the cumulated dry matter, we will explore the relationship between these two estimates. Thus, Eq. (9) can be rewritten as follows:
Substituting (6) in (11), we obtain:
In Eq. (12), it can be observed that when the RUE is estimated without considering respiration (usual RUE), a linear relationship as a function of time is not kept because the accumulated respiration and accumulated PAR are not constant over time. This clearly evidences that not considering respiration in the estimation of RUE is a misconception. In other words, if for some reason, it is required to estimate a RUE without considering respiration, it can never be constant.
Estimating RUE Under Completely Controlled Conditions – Using Thornley Model
This section assumes a constant RUE for each day, and all environmental conditions are constant, such as temperature, relative humidity, and soil water and nutritional conditions. Here, it is necessary to state some assumptions about the respiration process. We are using the classical approximation proposed by Thornley (1970, 1971) (Thornley 1970, 1971) where the respiration process \(\left({r}_{t}\right)\) was divided into growth \(\left({r}_{g}\right)\) and maintenance respiration \(\left({r}_{m}\right)\) as follows:
where m is the maintenance respiration rate (i.e., 0.03 g respirated by total dry matter (g)), and c is the growth respiration rate (i.e., 0.273 g respirated by assimilated (g)) (Amthor 2000; Penning de Vries and Van Laar 1982). Substituting (14) into (9), we obtain:
Therefore, a linear regression with no intercept between \(\frac{{W}_{t}+m\int {W}_{t}{d}_{t}}{\left(1-c\right)}\) and \({A}_{t}\) would be the constant RUE for the whole plant growth cycle. The integral \(\int {W}_{t}{d}_{t}\) would be calculated numerically using the observed values of \({W}_{t}\). Note that growth and maintenance respiration rates are considered constants throughout the plant growth cycle in this approach. This situation could be possible under greenhouse environment where the conditions are entirely controlled.
Estimating RUE Under Field Conditions – Using Thornley Model
In field conditions, respiration rates fluctuate and are predominantly influenced by temperature. The temperature-dependent nature of respiration implies that RUE may also be influenced by temperature variations. Thus, a more realistic model should consider variable respiratory rates and possibly variable RUE. Based on the classical respiration model, Eq. (7) is rewritten as follows:
An alternative way to estimate \({\Lambda }_{t}\) based on Eq. (20) is to consider a discrete model where the resolution of the climatic variables at least daily, as follows:
For the first day after emergence:
For the second day after emergence:
For the third day after emergence,
Rewriting in matrix notation the equations above, we have:
This can be generalized as follows:
where w is the cumulated weight vector with form \(\left[\begin{array}{c}{W}_{1}\\ \vdots \\ \begin{array}{c}{W}_{l}\\ \vdots \\ {W}_{h}\end{array}\end{array}\right]\) where \(d=\left\{1, 2, \dots ,h\right\}\) indicating the growth days until the harvest date h; L is a lower diagonal matrix with the form:
where \(j=\left\{\mathrm{0,1},\dots ,h-1\right\}\) indicates the columns and d indicates the rows, and m is a vector of size h with the form \(\left[\begin{array}{c}1-{m}_{0}\\ .\\ .\\ .\\ \prod_{j=0}^{d-1}\left(1-{m}_{j}\right)\\ .\\ .\\ .\\ \prod_{j=0}^{h-1}\left(1-{m}_{j}\right)\end{array}\right]\), and \({w}_{0}\) is the initial weight. The RUE corresponds to vector Λ of size h, indicating that there is one RUE for each growth day. The linear system of Eq. (31) can be solved by any linear algebra method and, in this way, estimate RUE.
An option for obtaining daily respiration rates is from a respiration rate measured under constant temperature conditions and then apply the rule of Q10 using the daily mean temperature to generate the daily growth and maintenance respiration rates, thus:
Estimating RUE Under Field Conditions from Frequent Biomass Samples – Using Thornley Model
To estimate the RUE from periodic biomass samples, we must assume that the RUE value between two short-time harvestings varies slightly and can be considered in practical terms as constant for that short time. Based on the model in matrix notation, this is equivalent to:
where,
where s corresponds to the harvest sampling, D is the number of days between the sampling s-1 and s, and \({1}_{D-1}\) is a vector of 1 with size D-1. Equation (33) can be estimated by any common method of matrix algebra.
Finally, as an option, although we consider it unrealistic, perhaps in some studies, it may be of interest, it would be to estimate a constant RUE for the entire crop cycle, for which Eq. (33) is rewritten as:
Because temperature affects the physiological processes involved in the crop growth and development, and consequently in the estimation of the RUE, The RUE values (RUEv) estimated per day or by sampling was transformed at constant temperature also using the Q10 rule as follows:
The base temperature was set at 15 °C and the mean temperature corresponds to the average temperature of the day or the sampling interval.
Calculations and graphs were made with R statistical software (R Core Team 2023).
Results
The calculated RUE, considering respiration processes throughout the crop season, exhibited noteworthy fluctuations over time. In experiment 1 (Fig. 2a), during the initial stages of crop growth, the RUE experienced a modest decline from 2.62 to 1.98 g MJ−1 at 39 DAE. Subsequently, there was a substantial increase in RUE, peaking at 7.08 g MJ−1 around 66 DAE. This peak in RUE coincided with the period of maximum tuber growth rate, occurring between 60 and 70 DAE. Following 70 DAE, as tuber filling stabilized, the RUE demonstrated a decline.
Radiation use efficiency – RUE (g MJ−1) at 15 ºC (left ordinate axis). RUE obtained by daily interpolation samples over time (days after emergence – DAE) (abscissa axis) and tuber dry weight (g m−2) (right ordinate axis) for the diploid cultivar ‘Colombia’ in six experiments. Experiment 1 is depicted in figure a, Experiment 2 in figure b, Experiment 3 in Figure c, Experiment 4 in Figure d, Experiment 5 in Figure e, and Experiment 6 in figure f. The continuous blue line represents the RUE values (RUEv) for each of the actual biomass samples, and the blue dotted line indicates the limits of confidence at 95%. The circles correspond to the RUE estimated for each day of crop growth obtained by interpolation. The black and orange dotted line represents the constant RUE for the whole crop season considering and without considering the respiration processes, respectively. The brown line represents the tuber growth over time
The period while the RUE increased coincided with the time of maximum LAI values reached by the crop (> 2.5) and these values decreased from 53 DAE to the end of the cycle. The reduction in RUE coincided with the crop senescence since the vegetative organs of the plant (stems and leaves) lost biomass from 66 DAE due to this natural process until the end of the cycle.
In experiment 2, the RUE reduced during the first 30 DAE from 2.48 to 2.19 g MJ−1. Then, the values increased until 55 DAE, reaching 3.65 g MJ−1. The values ranged between 2.53 and 3.79 g MJ−1 until day 70, when the RUE increased again to 5.86 g MJ−1 until the end of the cycle (Fig. 2b). The fact that the crop increased the RUE at the end of the cycle suggests that the crop was still in full growth and that the foliage sanitary conditions were adequate. Diploid potato crops end their senescence because of P. infestans. If this disease were permanently controlled, the crop would always remain green and photosynthetically active. In experiment 3, the initial reduction of RUE is delayed until 49 DAE. Then, the RUE increased from 0.90 to 4.61 g MJ−1 at 76 DAE. At the end of the cycle (83 DAE), the RUE achieved was 3.44 g MJ−1 (Fig. 2c).
The highest initial values of RUE were obtained in experiments 4, 5, and 6. Experiment 4 showed at the beginning of the emergence (9 DAE) a RUE value equal to 4.84 g MJ−1. With the canopy growth, the RUE dropped until tuber initiation at 46 DAE, reaching 1.60 g MJ−1, and the RUE values increased until it achieved the maximum RUE in this experiment (6.11 g MJ−1) at 50 DAE (Fig. 2d). With crop senescence, the RUE deceased until the final harvest.
The initial RUE in experiment 5 at 10 DAE was 9.91 g MJ−1. This initial value is excessively high and abnormal; this value could be explained by the fact that the small seedlings sampled in that initial growth phase were very small, had a couple of leaves, and were fully expanded without any restriction in the interception and absorption of received light energy. To 24 DAE, the RUE decreased dramatically, and it reached a value equal to 1.47 g MJ−1. At 31 DAE, when tuber initiation was recorded in this experiment, the RUE increased until 53 DAE (5.36 g MJ−1). The RUE obtained at the end of the crop cycle (73 DAE) was 4.18 g MJ−1 (Fig. 2e).
The initial RUE in experiment 6 was the highest among the six experiments (16.84 g MJ−1). The RUE notably decreased to 1.76 g MJ−1 at 17 DAE. The values started to increase at 24 DAE. At 58 DAE, the crop achieved 5.78 g MJ−1. This value was reduced to the end of the crop cycle, with a RUE equal to 4.33 g MJ−1 (Fig. 2f).
In general terms, it is possible to affirm that the diploid potato cultivar 'Colombia' during the first days of the emergence until 44 DAE, reached RUE values between 1.37 and 2.97. Then, when the tuberization process began, these values increased, and the crop achieved values between 1.66 and 7.08. Finally, at the end of the cycle, with the stabilization of the tubers filling and the leaves senescence, the RUE values decreased, reaching values between 0.95 and 4.33.
Figure 2 provides a representation of the RUE along with notable variations when accounting for respiration processes and the total biomass accumulation over time while assuming a constant RUE. In the absence of considering respiration processes, the RUE, calculated as the slope of the relationship between total cumulative biomass and cumulative photosynthetically active radiation (PAR) for each of the six experiments, was 1.63, 1.61, 1.26, 1.83, 2.00, and 1.72 g MJ−1 (depicted by the orange dotted line in Fig. 2). However, when incorporating respiration in the computation of a constant RUE throughout the entire crop cycle, consistently higher values emerged: 2.74, 2.93, 2.71, 3.46, 3.74, and 3.18 g MJ−1 for each experiment (represented by the black dotted line in Fig. 2).
It is possible to estimate the daily dry weight through interpolation processes from the dry weights obtained in biomass samples and the daily weather data. With this information, the RUE can be estimated based on Eq. (33), and the empty circles in Fig. 2 represent these estimations. If dry weight interpolation is not available, the following is to use the biomass samples data and apply Eq. (5).
Considering that the RUE is a frequently used parameter in the development of crop models and the crop models generate daily growth data, empty circles in Fig. 2 show the RUE obtained by daily interpolation, that is, the interpolated RUE for each growth day. This interpolation allows us to see the RUE trend and its variation throughout the growing cycle. The estimated RUE values in the first days of emergence are significantly higher. When plants are newly emerged, their entire leaf area is exposed and captures the most significant amount of solar radiation to continue their accelerated growth at that early stage. Then, RUE decreases with vegetative growth, and its value increases again when the tuber filling process starts. At the end of the cycle, with the canopy senescence, the plant loses the capacity of interception solar radiation, loses photosynthetic capacity, and respiratory processes are increased, and consequently, the RUE is reduced.
Discussion
The RUE has been widely used as the slope of the relationship between the total cumulated biomass and the intercepted radiation (Murchie et al. 2019; Stöckle and Kemanian 2009). That single value has been applied to explain the growth as a function of solar radiation throughout the crop cycle (see studies in potato crop in Table 2). The results obtained in this study show that the RUE values fluctuate with the plant age that determines the canopy extension and distribution, its radiation interception capacity, and the growth and maintenance respiration also vary with time. For this reason, employing a constant value to determine the efficiency in the use of radiation of an entire crop cycle may result in inaccuracies when predicting crop productivity and yield.
A constant RUE over the complete crop season does not consider the different factors that affect the crop depending on the developmental stage. As shown in Fig. 2, during the early stages of growth after emergence, the young and small leaves are fully capable of intercepting the incident radiation and involving it in the photosynthesis processes to produce biomass and its subsequent translocation. The younger the plants, the higher the plants ability to intercept radiation and the photosynthetic ability. For this reason, experiments 4, 5, and 6 have higher initial RUE values than in experiments 1, 2, and 3 because the biomass samples were harvested as early as possible. Thus, as discussed, RUE values are higher during the early stages. Besides, in this early developmental stage, the plant growth rates are high, in contrast to a low growth respiration rate, since the amount of existing biomass is also low. Likewise, the maintenance respiration rate of that young biomass is low because youthful tissues in full growth do not demand significant maintenance that implies energy costs. It is important to highlight that biomass samples in experiments 1, 2, and 3 started at 18, 6, and 8 DAE, respectively. And in experiments 4, 5, and 6, they started at the emergence day.
The potato cultivar¡ used in this study belong to Solanum phureja species, characterized by their short life cycle, short day adaptation, and diploid nature (2n = 2x = 24) (Ovchinnikova et al. 2011). Due to the short cycle and precocity, S. phureja cultivars growth fast (Ghislain et al. 2006; Machida-Hirano 2015), and at 24 DAE the tuber initiation started. This physiological stage changes the growth dynamics of the crop. The tuber filling constitutes in significant demand for photoassimilates. When the tubers start filling, the canopy is fully developed to intercept as much radiation as possible and involve it in the biomass production process. For this reason, the value of the RUE increases during this stage to contribute to supply the tuber carbohydrates demand.
At the end of the crop cycle of this type of fast-growing diploid cultivars, when the tuber filling stabilizes and the senescence process begins mainly due to sanitary factors, the RUE values are reduced because the leaves lose photosynthetic capacity. Besides, during senescence, the maintenance respiration rate is high (Loomis and Amthor 1999).
In this sense, it is crucial to highlight that using a unique or constant RUE for the entire crop cycle is a mistake. Using a single value leads to crop growth being overestimated or underestimated in its different development stages. Furthermore, in many scenarios aimed to simulate and improve the understanding of crop growth and development, the concept of RUE has been usually used as an important method (Bonhomme 2000).
More than 30 crop models are used to simulate potato growth (Fleisher et al. 2017; Raymundo et al. 2014; Saqib and Anjum 2021), and in some of these models, an unique constant RUE value is used to estimate the biomass increase. APSIM-Potato uses a constant RUE value of 1.44 g MJ−1, and this value is multiplied by adjustment factors to account for the effects of water and temperature stresses and atmospheric carbon dioxide concentration (Brown et al. 2011). The INFOCROP-Potato model uses a RUE of 3.50 g MJ−1 during vegetative and tuber growth (Singh et al. 2005). LINTUL Potato DSS uses a single RUE value for the potato complete crop season of 1.25 g MJ−1 (Haverkort et al. 2015). The SUBSTOR-Potato model uses two RUE values, 3.50 g MJ−1 from emergence to tuber initiation, and 4.00 g MJ−1 from tuber initiation to maturity (Griffin et al. 1993; Hoogenboom et al. 2018).
However, models such as NPOTATO (Wolf 2002) computes the daily growth as radiation interception multiplied by a specified RUE. The radiation interception in this model is calculated from incoming radiation and the fractional radiation interception. Then, RUE is corrected for sub-optimal temperatures and soil water contents and for a change in atmospheric CO2 concentration. This approach estimates the RUE considering better assumptions.
Understandably, the extensively used constant RUE concept, as the slope of the relationship between the total biomass and the solar radiation intercepted, is so widely accepted. This occurs because the adjustments are very good. For example, in most cases, the studies listed in Table 2, R2, are higher than 0.90. This allows us to infer an optimal fit, and it is assumed that the RUE could be single and constant for the entire crop cycle. Based on our research findings, the RUE is not a constant value as the respiration process leads to RUE values being variable in a non-linear way over time.
Conclusion
This study highlights that during the crop growth, the RUE is variable in the different stages of developing of the diploid potato crop. The rapid growth of these cultivars means that vital processes such as the synthesis of photoassimilates, their translocation, and maintenance and growth respiration processes are reflected in the radiation use efficiency. The RUE increases at the beginning of the tuberization process to around 40 DAE. Moreover, while the tuber filling is occurring, the maximum RUE values are reached around 60 DAE.
The simulation of the daily RUE throughout an interpolation process shows an important variation from the first day of the emergence to the end of the cycle that suggests that assuming a constant RUE for the entire growth period is an error. Moreover, the RUE calculations must include processes in which there is a loss of carbohydrates, such as respiration.
References
Allen, E.J., and R.K. Scott. 1980. An Analysis of Growth of the Potato Crop. Journal of Agricultural Science, Cam 94 (3): 582–606. https://doi.org/10.1016/s0065-2113(08)60914-1.
Amthor, Jeffrey S. 2000. The McCree-de Wit-Penning de Vries-Thornley Respiration Paradigms: 30 Years Later. Annals of Botany 86 (1): 1–20. https://doi.org/10.1006/anbo.2000.1175.
Bassham, James Alan and Lambers, Hans. “photosynthesis”. Encyclopedia Britannica, 2 Dec. 2023, https://www.britannica.com/science/photosynthesis
Bonhomme, Raymond. 2000. Beware of Comparing RUE Values Calculated from PAR vs Solar Radiation or Absorbed vs Intercepted Radiation. Field Crops Research 68 (3): 247–252. https://doi.org/10.1016/S0378-4290(00)00120-9.
Brown, H.E., N. Huth, and D. Holzworth. 2011. A Potato Model Built Using the APSIM Plant.Net Framework. MODSIM 2011 - 19th International Congress on Modelling and Simulation - Sustaining Our Future: Understanding and Living with Uncertainty 961–67. https://doi.org/10.36334/modsim.2011.b3.brown.
Burstall, Lindsay, and P.M. Harris. 1986. The Physiological Basis for Mixing Varieties and Seed ‘Ages’ in Potato Crops. The Journal of Agricultural Science 106 (2): 411–418. https://doi.org/10.1017/S0021859600064029.
Camargo, D.C., F. Montoya, M.A. Moreno, J.F. Ortega, and J.I. Córcoles. 2016. Impact of Water Deficit on Light Interception, Radiation Use Efficiency and Leaf Area Index in a Potato Crop (Solanum Tuberosum L.). Journal of Agricultural Science 154 (4): 662–673. https://doi.org/10.1017/S002185961500060X.
Campbell, G., and J. Norman. 2011. The Light Environment of Plant Canopies. An Introduction to Environmental Biophysics 247–78. https://doi.org/10.1007/978-1-4612-1626-1_15.
Condori, Bruno, Pablo Mamani, Ruben Botello, Fernando Patiño, André Devaux, and Jean François Ledent. 2008. Agrophysiological Characterisation and Parametrisation of Andean Tubers: Potato (Solanum Sp.), Oca (Oxalis Tuberosa), Isaño (Tropaeolum Tuberosum) and Papalisa (Ullucus Tuberosus). European Journal of Agronomy 28 (4): 526–540. https://doi.org/10.1016/j.eja.2007.12.002.
De la Casa, A., G. Ovando, L. Bressanini, J. Martínez, and A. Rodríguez. 2011. Eficiencia en el uso de la radiación en papa estimada a partir de la cobertura del follaje. Agriscientia 28: 21–30.
de Wit, C. 1959. Potential Photosynthesis of Crop Surfaces. Netherlands Journal of Agricultural Science 7 (2): 141–149.
Dewar, Roderick C. 1996. The Correlation between plant growth and intercepted radiation: an interpretation in terms of optimal plant nitrogen content. Annals of Botany 78 (1): 125–136. https://doi.org/10.1006/anbo.1996.0104.
Evans, John R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. https://doi.org/10.1109/LSP.2017.2723724.
Fleisher, D., B. Condori, R. Quiroz, A. Alva, S. Asseng, C. Barreda, M. Bindi, K. Boote, R. Ferrise, A. Franke, P. Govindakrishnan, D. Harahagazwe, G. Hoogenboom, S. Naresh Kumar, P. Merante, C. Nendel, J. Olesen, P. Parker, D. Raes, R. Raymundo, A. Ruane, C. Stockle, I. Supit, E. Vanuytrecht, J. Wolf, and P. Woli. 2017. A potato model intercomparison across varying climates and productivity levels. Global Change Biology 23 (3): 1258–1281. https://doi.org/10.1111/gcb.13411.
Gallo, K.P., C.S.T. Daughtry, and C.L. Wiegand. 1993. Errors in measuring absorbed radiation and computing crop radiation use efficiency. Agronomy Journal 85 (6): 1222–1228. https://doi.org/10.2134/agronj1993.00021962008500060024x.
Ghislain, M., D. Andrade, F. Rodríguez, R.J. Hijmans, and D.M. Spooner. 2006. Genetic analysis of the cultivated potato solanum tuberosum L. phureja group using RAPDs and nuclear SSRs. Theoretical and Applied Genetics 113 (8): 1515–1527. https://doi.org/10.1007/s00122-006-0399-7.
Gordon, R., D.M. Brown, and M.A. Dixon. 1997. Estimating potato leaf area index for specific cultivars. Potato Research 40 (3): 251–266. https://doi.org/10.1007/BF02358007.
Griffin, T. S., Johnson, B. S., & Ritchie, J. T. 1993. A Simulation Model for Potato Growth and Development:. SUBSTOR - Potato Version 2.0. IBSNAT. Research Report Series 02. Dept. of Agronomy and Soil Science, College of Tropical Agriculture and Human Resources, Univ. of Hawaii, Honolulu, HI.
Guerrero, Ricardo. 1998. Fertilización de Cultivos En Clima Frío. Second edition. Bogotá D.C., Colombia: Monomeros Colombo Venezolanos S.A.
Haverkort, A.J., A.C. Franke, J.M. Steyn, A.A. Pronk, D.O. Caldiz, and P.L. Kooman. 2015. A robust potato model: LINTUL-Potato-DSS. Potato Research 58 (4): 313–327. https://doi.org/10.1007/s11540-015-9303-7.
Hoogenboom, G., C.H. Porter, V. Shelia, K.J. Boote, U. Singh, W. Pavan, F.A.A. Oliveira, L.P. Moreno-Cadena, T.B. Ferreira, J.W. White, J.I. Lizaso, D.N.L. Pequeno, B.A. Kimball, P.D. Alderman, K.R. Thorp, S.V. Cuadra, M.S. Vianna, F.J. Villalobos, W.D. Batchelor, S. Asseng, M.R. Jones, A. Hopf, H.B. Dias, L.A. Hunt, and J.W. Jones. 2018. Decision Support System for Agrotechnology Transfer (DSSAT) Version 4.7.2 (www.DSSAT.net). DSSAT Foundation, Gainesville, Florida, USA.
Jefferies, R.A., and D.K.L. Mackerron. 1989. Radiation interception and growth of irrigated and droughted potato (Solanum Tuberosum). Field Crops Research 22 (2): 101–112. https://doi.org/10.1016/0378-4290(89)90061-0.
Jing, Qi., Sjaak J.G. Conijn, Raymond E.E. Jongschaap, and Prem S. Bindraban. 2012. Modeling the productivity of energy crops in different agro-ecological environments. Biomass and Bioenergy 46: 618–633. https://doi.org/10.1016/j.biombioe.2012.06.035.
Khurana, S.C., and J.S. McLaren. 1982. The influence of leaf area, light interception and season on potato growth and yield. Potato Research 25 (4): 329–342. https://doi.org/10.1007/BF02357290.
Kooman, P.L., and R. Rabbinge. 1996. An analysis of the relation between dry matter allocation to the tuber and earliness of a potato crop. Annals of Botany 77: 235–242.
Loomis, R.S., and J.S. Amthor. 1999. Yield potential, plant assimilatory capacity, and metabolic efficiencies. Crop Science 39 (6): 1584–1596.
Loomis, R.S., and P.A. Gerakis. 1975. Productivity of Agricultural Ecosystems. In Photosynthesis and productivity in different environments, ed. J.P. Cooper, 145–72. Aberystwyth, UK: Cambridge University Press.
Loomis, R. S., & Williams, W. A. 1963. Maximum Crop Productivity: An Estimate. Crop Science, 3(1), 67–72. https://doi.org/10.2135/cropsci1963.0011183X000300010021x
Machida-Hirano, Ryoko. 2015. Diversity of potato genetic resources. Breeding Science 65 (1): 26–40. https://doi.org/10.1270/jsbbs.65.26.
McCree, K.J. 1971. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agricultural Meteorology 9 (C): 191–216. https://doi.org/10.1016/0002-1571(71)90022-7.
McCree, K.J. 1982. Maintenance requirements of white clover at high and low growth rates 1. Crop Science 22 (2): 345–351. https://doi.org/10.2135/cropsci1982.0011183x002200020035x.
Monsi, Masami, and Tosiro Saeki. 1953. Über den lichtfaktor in den pflanzengesellschaften und seine bedeutung für die stoffproduktion. Japanese Journal of Botany 14: 22–52.
Monteith, J.L. 1963. Light Distribution and Photosynthesis in Field Crops. Annals of Botany 29 (113): 17–37.
Monteith. 1972. Solar Radiation and Productivity in Tropical Ecosystems Author ( s ): J.L . Monteith Source : Journal of Applied Ecology , Vol . 9 , No . 3 ( Dec ., 1972 ), Pp . 747–766 Published by : British Ecological Society Stable URL : http://www.jstor.org/stable/. Society 9(3):747–66
Murata, Yoshio. 1981. Dependence of Potential Productivity and Efficiency for Solar Energy Utilization on Leaf Photosynthetic Capacity in Crop Species. Japan. Jour. Crop Sci. 50 (2): 223–232. https://doi.org/10.1626/jcs.50.223.
Murchie, Erik H., Alexandra Townsend, and Matthew Reynolds. 2019. Crop Radiation Capture and Use Efficiency. In Crop Science, ed. R. Savin and G. Slafer, 73–106. New York, USA: Springer.
Niinemets, Ülo. 2010. A Review of Light Interception in Plant Stands from Leaf to Canopy in Different Plant Functional Types and in Species with Varying Shade Tolerance. Ecological Research 25 (4): 693–714. https://doi.org/10.1007/s11284-010-0712-4.
Nishibe, Sachio, Masato Satoh, Motoyuki Mori, Akihiro Isoda, and Kimio Nakaseko. 1989. Effects of climatic conditions on intercepted radiation and some growth parameters in potato. Japanese Journal of Crop Science 58 (2): 171–179.
Nyawade, Shadrack O., Nancy N. Karanja, Charles K. K. Gachene, Harun I. Gitari, Elmar Schulte-Geldermann, and Monica L. Parker. 2019. Intercropping optimizes soil temperature and increases crop water productivity and radiation use efficiency of rainfed potato. American Journal of Potato Research 96 (5): 457–471. https://doi.org/10.1007/s12230-019-09737-4.
Ovchinnikova, Anna, Ekaterina Krylova, Tatjana Gavrilenko, Tamara Smekalova, Mikhail Zhuk, Sandra Knapp, and David M. Spooner. 2011. Taxonomy of cultivated potatoes (Solanum Section Petota: Solanaceae). Botanical Journal of the Linnean Society 165 (2): 107–155. https://doi.org/10.1111/j.1095-8339.2010.01107.x.
Patiño, Jenniffer. 2014. Análisis Funcional Del Crecimiento y Desarrollo de Variedades de Papa Criolla (Solanum Phureja Juz et Buk.) Bajo El Efecto de Densidades de Siembra.” Maestría en Ciencias Agrarias. Facultad de Ciencias Agrarias. Universidad Nacional de Colombia - Sede Medellín.
Penning de Vries, F., and H. Van Laar. 1982. Simulation of Plant Growth and Crop Production. Wageningen, The Netherlands: Pudoc.
R Core Team. 2023. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Raymundo, R., Senthold Asseng, Davide Cammarano, and Roberto Quiroz. 2014. Potato, sweet potato, and yam models for climate change: a review. Field Crops Research 166: 173–185. https://doi.org/10.1016/j.fcr.2014.06.017.
Rezig, Mourad, Ali Sahli, Mohamed Hachicha, Faycel Ben Jeddi, and Youssef Harbaoui. 2013a. Light interception and radiation use efficiency from a field of potato (Solarium Tuberosum l.) and Sulla (Hedysarum Coronarium l.) Intercropping in Tunisia. Asian Journal of Crop Science 5 (4): 378–392. https://doi.org/10.3923/ajcs.2013.378.392.
Rezig, Mourad, Ali Sahli, Mohamed Hachicha, Faycel Ben Jeddi, and Youssef Harbaoui. 2013b. Potato (Solanum Tuberosum L.) and bean (Phaseolus Vulgaris L.) in sole intercropping: Effects on light interception and radiation use efficiency. Journal of Agricultural Science 5 (9): 65–77. https://doi.org/10.5539/jas.v5n9p65.
Ritchie, J.T., T.S. Griffin, and B.S. Johnson. 1995. SUBSTOR: Functional model of potato growth, development and yield. In Modelling and Parameterization of the Soil-Plant-Atmosphere System: A Comparison of Potato Growth Models, ed. P. Kabat, B. Marshall, B.J. van der Broek, J. Vos, and H. van Keulen, 401–35. Wageningen Pers: Wageningen.
Saldaña-Villota, Tatiana M., and José Miguel. Cotes-Torres. 2020. Functional growth analysis of diploid potato cultivars (Solanum Phureja Juz. et Buk.). Revista Colombiana De Ciencias Hortícolas 14 (3): 402–415. https://doi.org/10.17584/rcch.2020v14i3.10870.
Sale, P.J.M. 1974. Productivity of vegetable crops in a region of high solar input. III. Carbon balance of potato crops. Functional Plant Biology 1 (2): 283. https://doi.org/10.1071/pp9740283.
Saqib, M., and M. Anjum. 2021. Applications of decision support system: A case study of solanaceous vegetables. Phyton - International Journal of Experimental Botany 90 (2): 331–352. https://doi.org/10.32604/phyton.2021.011685.
Sinclair, Thomas, and Russell Muchow. 1999. Radiation use efficiency. Advances in Agronomy 65: 215–265. https://doi.org/10.1016/s0065-2113(08)60914-1.
Singh, J., P. Govindakrishnan, S. Lal, and P. Aggarwal. 2005. Increasing the efficiency of agronomy experiments in potato using INFOCROP-POTATO Model. Potato Research 48 (3–4): 131–152. https://doi.org/10.1007/BF02742372.
Smith, Nicholas G., and Jeffrey S. Dukes. 2013. Plant respiration and photosynthesis in global-scale models: Incorporating acclimation to temperature and CO2. Global Change Biology 19 (1): 45–63. https://doi.org/10.1111/j.1365-2486.2012.02797.x.
Spitters, C. 1987. An analysis of variation in yield among potato cultivars in terms of light absorption, light utilization and dry matter partitioning. Acta Horticulturae 214: 71–84. https://doi.org/10.17660/actahortic.1988.214.5.
Squire, G. (1990). The physiology of tropical crop production (First edit). C.A.B. International. Oxon, UK. https://books.google.com.co/books/about/The_Physiology_of_Tropical_Crop_Producti.html?id=mQDxAAAAMAAJ&redir_esc=y
Stöckle, Claudio O., and Armen R. Kemanian. 2009. Crop radiation capture and use efficiency. Crop Physiology,145–70. https://doi.org/10.1016/b978-0-12-374431-9.00007-4
Stöckle, Claudio O., Armen R. Kemanian, and Cristián Kremer. 2008. On the Use of Radiation- and Water-Use Efficiency for Biomass Production Models. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes, ed. L.R. Ahuja, V.R. Reddy, S.A. Saseendran, and Y. Qiang, 39–58. Wisconsin: Madison.
Thornley, J.H.M. 1970. Respiration, growth and maintenance in plants. Nature 227: 304–305.
Thornley, J.H.M. 1971. Energy, respiration, and growth in plants. Annals of Botany 35 (4): 721–728. https://doi.org/10.1093/oxfordjournals.aob.a084519.
Thornley, J.H.M. 1977. Growth, maintenance and respiration: A re-interpretation. Annals of Botany 41 (6): 1191–1203. https://doi.org/10.1093/oxfordjournals.aob.a085409.
Thornley, J.H.M. 2011. Plant growth and respiration re-visited: Maintenance respiration defined it is an emergent property of, not a separate process within, the system and why the respiration: photosynthesis ratio is conservative. Annals of Botany 108 (7): 1365–1380. https://doi.org/10.1093/aob/mcr238.
Tjoelker, Mark G., Jacek Oleksyn, and Peter B. Reich. 2001. Modelling respiration of vegetation: Evidence for a general temperature-dependent Q10. Global Change Biology 7 (2): 223–230. https://doi.org/10.1046/j.1365-2486.2001.00397.x.
Trapani, N., A.J. Hall, V.O. Sadras, and F. Vilella. 1992. Ontogenetic changes in radiation use efficiency of sunflower (Helianthus Annuus L.) crops. Field Crops Research 29 (4): 301–316. https://doi.org/10.1016/0378-4290(92)90032-5.
Van Oijen, M. 1991. Light use efficiencies of potato cultivars with late blight (Phytophthora Infestans). Potato Research 34 (2): 123–132. https://doi.org/10.1007/BF02358033.
Warren Wilson, J. 1967. Ecological data on dry-matter production by plants and plant communities. In Collection and Processing of Field Data, ed. E. Bradley and O. Denmead, 77–123. John Willey.
Weraduwage, Sarathi M., Jin Chen, Fransisca C. Anozie, Alejandro Morales, Sean E. Weise, and Thomas D. Sharkey. 2015. The Relationship between Leaf Area Growth and Biomass Accumulation in Arabidopsis Thaliana. Frontiers in Plant Science 6 (APR): 1–21. https://doi.org/10.3389/fpls.2015.00167.
Wolf, Joost. 2002. Comparison of two potato simulation models under climate change. i. model calibration and sensitivity analyses. Climate Research 21: 173–186.
Zhou, Zhenjiang, Mathias Neumann Andersen, and Finn Plauborg. 2016. Radiation interception and radiation use efficiency of potato affected by different n fertigation and irrigation regimes. European Journal of Agronomy 81: 129–137. https://doi.org/10.1016/j.eja.2016.09.007.
Zhou, Zhenjiang, Finn Plauborg, Kristian Kristensen, and Mathias Neumann Andersen. 2017. Dry matter production, radiation interception and radiation use efficiency of potato in response to temperature and nitrogen application regimes. Agricultural and Forest Meteorology 232: 595–605. https://doi.org/10.1016/j.agrformet.2016.10.017.
Zhu, Xin Guang, Stephen P. Long, and Donald R. Ort. 2008. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Current Opinion in Biotechnology 19 (2): 153–159. https://doi.org/10.1016/j.copbio.2008.02.004.
Funding
Open Access funding provided by Colombia Consortium
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saldaña-Villota, T.M., Cotes-Torres, J.M. Fluctuations in Radiation Use Efficiency Throughout the Growth Cycle in Diploid Potato Crop. Am. J. Potato Res. (2024). https://doi.org/10.1007/s12230-023-09938-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12230-023-09938-y