Abstract
Most hemiparasitic plants are generalists with a broad host range, but they grow better in the presence of some plant species than with others. In mixed communities of hosts, hemiparasites prefer some hosts over others, but it is not yet known if hemiparasite roots can distinguish between the roots of different plant species and show directed growth (host tropism). We performed host choice experiments, exposing seedlings of Rhinanthus alectorolophus in agar plates simultaneously to seedlings of grass and legume species known to be hosts of good or poor quality for the parasite, and measured directed root growth and haustoria formation. Parasite roots did not show directed growth towards the roots of a good compared to a poor host species within a host functional group. However, parasite roots grew more strongly in the direction of legume than grass roots. The probability to form haustoria with host roots did not differ between host species, and microscopy revealed that functional haustoria were formed even with a very poor host, the grass Anthoxanthum odoratum. Our results show that growth experiments in agar plates are a suitable approach to study early host choice of hemiparasites. Our finding that hemiparasites can (initially) form functional haustoria even with very poor hosts emphasizes that the quality of a plant species as a host depends on several independent processes, including early host recognition, haustoria formation, resource supply and competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic plants attach to their host plants with specialized organs (haustoria) and extract water, nutrients and carbohydrates (Kuijt 1969; Rümer et al. 2007; Jiang et al. 2010; Těšitel 2016). In contrast to holoparasites, which obtain all resources from their hosts, 90% of the known parasitic plant species are hemiparasites and produce at least a part of their carbon requirements through their own photosynthesis (Heide-Jørgensen 2008). Studies based on excavations of parasite root systems tracing back host roots (e.g. Weber 1976; Gibson and Watkinson 1989) or identifying host species by DNA barcoding (Holá et al. 2017) suggested that many hemiparasites are generalists, forming haustorial connections to roots of most plant species growing nearby. However, haustoria are not necessarily functional (Rümer et al. 2007; Holá et al. 2017) and parasites have been found to form haustoria even with dead roots, stones or plastic cables (Kuijt 1969; Pate et al. 1990). Experiments have demonstrated that plant species differ strongly in their quality as hosts for hemiparasites (e.g. De Hullu 1984; Matthies 2017, 2021). Differences in host quality can be due to defence mechanisms, as some plant species are able to block the formation of haustoria (Cameron and Seel 2007; Rümer et al. 2007; Yoder and Scholes 2010). Moreover, the quality of a species as a host for a hemiparasitic plant may also depend on the quality and quantity of resources supplied (Keith et al. 2004; Hautier et al. 2010; Sandner and Matthies 2018) and on the strength of competition with the host plant for light (Matthies 1995). Many hemiparasites are annuals whose seedlings have to establish connections to suitable hosts every year, and one could thus expect selection for the ability to distinguish good from poor host species early in their life.
Host selection by hemiparasites is still poorly understood. It has been found that the number of haustoria with different host species is not proportional to the abundance of the roots of the hosts in the soil (Gibson and Watkinson 1989; Suetsugu et al. 2008). Moreover, in pot experiments using mixtures of different hosts, Rhinanthus alectorolophus suppressed the growth of some species (particularly legumes) more strongly than expected based on its suppression of the same species in monoculture (Sandner and Matthies 2018), which could be related to the generally good quality of legumes as hosts for Rhinanthus species (de Hullu 1984; Rümer et al. 2007; Matthies 2021). There is thus evidence for some degree of active host choice by root hemiparasites, but it is not known if their roots grow randomly until they encounter suitable host roots (Oesau 1975), or if they actively forage for suitable hosts (‘host tropism’, Williams 1961; Mutuku et al. 2021).
Mechanisms allowing early host recognition by hemiparasites have been explored by growing parasites in agar plates in the presence of host plants or just of solutions of certain chemical compounds (Atsatt et al. 1978; Yoder 1997; Albrecht et al. 1999). Until now, a large number of substances have been identified which mediate host recognition in hemiparasites (Clarke et al. 2019; Mutuku et al. 2021). However, these substances only induce haustoria formation once the parasite root is in contact with a potential host root – the earlier step of host localization is far less understood (Mescher et al. 2009). In the stem parasite Cuscuta, foraging for suitable host species is guided by volatiles (Runyon et al. 2006) and light cues (reviewed by Clarke et al. 2019). In Striga and Orobanche, roots of seedlings grow in the direction of suitable hosts (Williams 1961; Whitney and Carsten 1981) and chemical cues have recently been identified (Krupp et al. 2021). This step is crucial for these parasite species, because they are both obligate parasites with minute seeds which would quickly die without attachment to a host. By contrast, seedlings of facultative hemiparasites can survive and even produce some flowers without a host (Kuijt 1969; Heide-Jørgensen 2008). Facultative hemiparasites might also initially show undirected root growth, but later show increased growth in the vicinity of the roots of suitable hosts (Oesau 1975; Atsatt 1983). It is not known if chemical cues are used by root hemiparasites to direct root growth towards the roots of certain plant species and to discriminate between hosts of good or poor quality. Here, we used host choice experiments in agar to differentiate between random root growth of a root hemiparasite and active foraging for host roots.
To study early host choice of the facultative root hemiparasite Rhinanthus alectorolophus, we grew a hemiparasite seedling together with seedlings of two different host species and studied whether root growth was directed (host tropism) towards one of the hosts and whether there were differences in haustoria formation. We hypothesized that R. alectorolophus would prefer legumes to grasses, and good to poor hosts. Moreover, we expected that R. alectorolophus would not be able to form functional haustoria with very poor host species that did not increase parasite size in prior experiments.
Material and Methods
Study Species
Rhinanthus alectorolophus (Scop.) Pollich is an annual hemiparasite from the Orobanchaceae family growing in nutrient-poor to mesotrophic grasslands of Central and Eastern Europe (Hartl 1974). Seeds of R. alectorolophus were collected in summer 2019 in a large natural population near Großalmerode in northern Hesse, Germany.
As host species we used grasses and legumes identified as good or poor hosts for R. alectorolophus based on parasite growth in previous experiments (Sandner and Matthies 2017; Matthies 2021); a good grass host (Dactylis glomerata L.), a poor grass host (Anthoxanthum odoratum L.), a good legume host (Medicago sativa L.) and a poor legume host (Anthyllis vulneraria L.). In the following, we will refer to the host species only by their genus name. Grown with the two good hosts the parasite reached a biomass of 200–1,000 mg whereas grown with the two poor hosts they grew hardly more than without a host (20–90 mg; Sandner and Matthies 2017; Matthies 2021). No defence mechanism has been reported to explain the poor host quality of Anthyllis and Anthoxanthum. The four host species all co-occur with R. alectorolophus in grasslands. Seeds of the hosts were obtained from a commercial supplier (Appels Wilde Samen, Darmstadt, Germany).
Experimental Design
Parasite seeds germinated in Petri dishes on wet filter paper at 4 °C after a stratification period of ca 3 months. When the radicle had emerged, seedlings where transferred to separate Petri dishes and kept at 4 °C (12 h light) for two weeks to form cotyledons. Every week, seedlings that had recently unfolded their cotyledons were used for a new set of host-choice experiments. Seeds of the host species were freshly germinated every week on wet filter paper at 22 °C.
To observe parasite root growth, parasites and hosts were grown in square 12 × 12-cm transparent plastic Petri dishes. Three notches of 5 mm diameter each were cut at a distance of 1.5 cm into one side of each dish and its cover (Fig. 1a). Petri dishes were filled 7 mm high with 100 mL of agar (0.8%), which in half of the experiments was enriched with a nutrient solution (Knop’s solution, 1.44 g·L−1 Ca(NO3) [4 H2O], 0.25 g·L−1 MgSO4 [7 H2O], 0.25 g·L−1 KH2PO4, 0.25 g·L−1 KNO3, traces of FeSO4 [7 H2O]).
(a) Schematic illustration of the experimental units. Two host and one parasite seedling were planted in Petri dishes filled with agar, with the possibility to grow out of the dish through holes. (b) Measurement of directed parasite root growth: the right and left red lines illustrate the measurement of lateral extension of the parasite root system in the direction of the two host species. The dotted red line in (a) and (b) indicates the midline of the Petri dish, where the parasite had been planted.
Three parallel lines with 1.5 cm distance were cut into the agar, and the roots of parasite and host seedlings were planted into the agar (Fig. 1a) in four different host choice combinations. In each Petri dish, one Rhinanthus seedling was placed between seedlings of (1) a good and a poor grass host; (2) a good and a poor legume host; (3) a good grass and a good legume host; or (4) a good grass and a poor legume host; for the classification of hosts as good or poor, see section ‘study species’. In each host combination, each of the two host species was planted either on the right or left side of the parasite in equal numbers to avoid any confounding of host species and direction (e.g. due to small differences in temperature or light). Every week, a new set with 6–10 replicates of each host combination was started, depending on the number of parasite seedlings available, 145 Petri dishes in total, of which 27 had to be excluded from the analysis because one of the three seedlings died. During the first three weeks (= 72 plates), seedlings were planted in agar enriched with nutrients. As growth of algae in these plates reduced the visibility of roots, agar without nutrients was used for the following three weeks (= 73 plates). The root length of the two host seedlings at the time of planting was measured as a covariate.
After planting, the front of the Petri dishes was covered with aluminium foil to protect the roots from light. The plates were kept at an angle of 45° with the holes facing up in a climate chamber (19°C, 12 h light from LED plant growth lamps, Floris 270, Alexander Neusius, Schiffweiler, Germany) and covered with plastic foil to prevent desiccation. After three weeks, the plates were placed on a light table and root growth was documented by standardized vertical photos. We terminated the experiment after three weeks, because host roots were increasingly overlapping and growing on both sides of the parasites, as well as along the bottom of the plates, so that directed growth of the parasite could no longer be studied (Fig. S1 in the Electronic supplementary material). The presence of haustoria formed with each host individual was noted, and several haustoria formed with Anthoxanthum were isolated and conserved in FPA (37% formalin, 5 parts; propionic acid, 5 parts; 50% ethanol, 90 parts).
For microscopic preparation, haustoria were transferred to 70% ethanol for 24 h before soaking in 100% LR White Resin (London Resin, Agar Scientific Ltd, Essex, United Kingdom) at 4°C for 24 h. Samples were embedded in white resin at 60°C for 24 h, and complete serial sections of 4 µm were prepared with a microtome (2065 Supercut, Leica Instruments GmbH, Nussloch, Germany). Cuttings were stained with Toluidine blue (10 g sodium tetraborate and 10 g Toluidine blue O in 1 L distilled water) and mounted in EUKITT neo spezial (O. Kindler, ORSAtec GmbH, Bobingen, Germany). Cuttings were inspected for xylem continuity between parasite and host under a light microscope.
Data Analysis
From the photos of the root systems after three weeks of growth, the lateral growth of the parasite root orthogonal to the mid-line of the Petri dish towards each host (Fig. 1b) was measured using the software ImageJ (Schneider et al. 2012). This variable proved to be a relatively robust measure of directed growth of the parasite roots. In contrast to other potential variables (minimum distance to the next host root, number of intersections of host and parasite roots) it depends only on the growth of the parasite root and cannot be falsely inflated by host roots growing towards or even across the parasite root. Root growth is not only lateral but is three-dimensional (growth in the third dimension being relatively restricted by the thin layer of agar). Although parasites and hosts were planted at a distance of only 1.5 cm, the parasite root could easily grow 5 cm to one side without encountering a host root.
Directed root growth was analysed by treating the data from each host combination as from a randomized blocks design with Petri dish as a block factor and the host species as factor of influence. In additional analyses, the initial length of a host root, the presence of nutrients in the agar (yes or no), and the interaction of nutrients and host species were included as fixed factors and removed when not significant.
To account for the possibility that parasite roots grew more strongly in the direction where the distance to a host root was greater, we measured from the same photos the lateral extension of the root system of both host species (see Fig. S1 for examples). The effects of host species and nutrients on the lateral extension of the host root system were analysed by ANOVA including Petri dish as a block factor. The effect of the lateral extension of a host root system on the directed root growth of the parasite was analysed by linear regression for all species together, with Petri dish identity included as a block factor. In further analyses, the lateral extension of the host root systems was included as a covariate in the models described above that analyse the effect of host species on the directed root growth of the parasite.
The difference between host species in the proportion of individuals with which the parasite formed haustoria was tested with a chi-square test. All analyses were performed in SPSS version 28.
Results
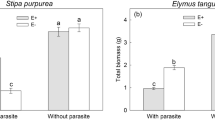
After three weeks, the lateral growth of the parasite root did not differ between hosts classified as good or poor in combination with the two grasses (F1, 30 = 0.218, P = 0.644, Fig. 2a) or the two legumes (F1, 30 = 2.68, P = 0.112, Fig. 2b). However, in the combinations of a grass and a legume, there was a tendency of stronger parasite root growth towards the good legume host Medicago (F1, 31 = 3.39, P = 0.075, Fig. 2c), and a much stronger growth towards the poor legume host Anthyllis (F1, 24 = 6.92, P = 0.015, Fig. 2d). The directed growth of parasite roots towards Anthyllis was in some cases already visible after one week of growth, together with the initiation of haustoria (Fig. 3). Initial root length of a host seedling had no effect on root growth of the parasite (all P > 0.20), except in the combination of a good grass host (Dactylis) and a good legume host (Medicago), where the parasite grew more in the direction of the longer host root (F1, 30 = 4.27, P = 0.047). Corrected for the difference in initial root length the difference between the grass and the legume was no longer significant (F1, 30 = 0.13, P = 0.724).
The presence of nutrients in the agar did not influence parasite root growth (all P > 0.05), except in the combination of a good and a poor legume, where overall lateral growth of parasite roots was increased in plates without added nutrients (F1, 28 = 4.23, P = 0.049). The lateral extension of the root system of a host, a possible confounding factor for directed parasite root growth, was influenced by nutrient addition (F1, 113 = 239.6, P < 0.001), host species (F3, 113 = 71.2, P < 0.001), and their interaction (F3, 113 = 117.4, P < 0.001). The root systems of Medicago and Anthyllis became wider in the presence of nutrients, while that of Dactylis became narrower and that of Anthoxanthum did not change (Fig. S2 in the Electronic supplementary material). However, the directed root growth of the hemiparasite was not at all related to the lateral extension of the root system of a host (R2 = 0.004, P = 0.507, d.f. = 118, Fig. S3), and accounting for variation in the extension of the host root system did not qualitatively change the effects of directed parasite root growth (Medicago vs Dactylis: F1, 30 = 2.98, P = 0.095; Anthyllis vs Dactylis: F1, 23 = 4.93, P = 0.037).
During the three-week experiments, the parasites formed haustoria with 22.7% of the host plants, but this proportion did not differ between host species (Chi2 = 2.32, d.f. = 3, P = 0.491). In the microscopic analyses of haustoria formed with the poor host Anthoxanthum, a functional connection between parasite and host root was found (Fig. 4).
Discussion
During the study period of three weeks after planting, the parasite seedlings did not show directed growth in the direction of plant species which had been found to be good hosts in previous pot experiments. However, when the two host seedlings belonged to two different functional groups, Rhinanthus roots grew preferably in the direction of the legume.
There may be several reasons why parasite roots did not show directed growth in the direction of particularly suitable host plants. First, the natural selection on the ability to recognize good host species may not be very strong, as hemiparasites usually grow in very diverse grassland habitats (Těšitel et al. 2015), where it is easy to encounter the roots of several suitable host species by chance. Contact to good hosts can then be strengthened by forming more lateral roots in the vicinity of existing haustoria, as was observed for the hemiparasite Melampyrum arvense (Oesau 1975). Second, directed growth towards certain species may not be advantageous, as the quality of a species as a host can depend on environmental conditions and phenological stage of a parasite. For example, differences in mortality of parasite seedlings with different host species were not related to differences in final biomass produced with the same host species (Sandner and Matthies 2018). Although haustoria formation comes with energetic costs, and ‘feeding mistakes’ by attaching haustoria to non-host species may be detrimental to a parasite (Atsatt 1977), this is far less problematic for facultative hemiparasites than for obligate parasites. The positive effects of bet hedging by attaching to all hosts available (Atsatt 1983) may thus outweigh the costs of some malinvested haustoria.
The observation of functional haustoria with the grass Anthoxanthum odoratum is remarkable, as this species is a very poor host for R. alectorolophus (Matthies 2021), and parasites grown with this plant were on average not larger than parasites grown without a host (Sandner and Matthies 2017). We had thus expected some kind of defence mechanism, like blocking the formation of haustoria by incrustations of tannin or lignin (e.g. Trifolium subterraneum and Leucanthemum vulgare – Govier 1966; Rümer et al. 2007) or programmed cell death (Plantago lanceolata, Rümer et al. 2007). However, the haustorium showed an intact connection (xylem bridge) between the xylem of parasite and host (Fig. 4). The poor host quality of Anthoxanthum must thus be due to other factors, like reduced supply of the parasite with resources, or resistance of parasite attack even after successful haustoria formation (Yoder and Scholes 2010). In the case of Anthoxanthum this could be related to its production of coumarins (Yamamoto and Fujii 1997; Tava 2001). Our results do not confirm allelopathic effects of Anthoxanthum on root growth as described by Yamamoto and Fujii (1997), as parasite roots grew as much in the direction of Anthoxanthum roots as in the direction of the other grass, Dactylis. However, coumarins are known as a chemical defence against herbivory (Berenbaum 1983; Rehman et al. 2012), and the excretion of hydroxylated coumarins has been linked to resistance against parasitism by Orobanche cernua (Serghini et al. 2001). Although the mechanism remains unresolved, our results show that a plant species can be a very poor host although functional haustoria are formed at the seedling stage. As the performance of hemiparasites is related to the number of haustoria formed (e.g. Rowntree et al. 2014), parasites may form fewer further haustoria after an initial contact with poor hosts.
The directed growth of parasite roots towards legume roots is in line with the results of an experiment in which R. alectorolophus suppressed the growth of legumes in mixtures of hosts more strongly than expected based on its growth in monocultures, suggesting that legumes were preferred to other hosts in mixtures (Sandner and Matthies 2018). A possible reason for this preference for legumes may be that their roots are particularly easy to recognize. Among the host recognition substances inducing haustoria formation are flavonoids (Albrecht et al. 1999; Yoder 2001), which are actively secreted by legumes to attract mutualistic Rhizobia (Albrecht et al. 1999; Oldroyd 2013). A recognition of Rhizobium-attracting substances by hemiparasites could thus explain the preference in mixtures of legumes over other host species suggested by pot experiments (Sandner and Matthies 2018). There are already examples showing that cues emitted by a plant can be read by ‘friends’ as well as ‘enemies’, and eavesdropping on the communication between plants and beneficial symbionts is also a likely explanation for the induction of parasite germination by strigolactones (Karban 2021).
Legumes may not only be easy to recognize, but their recognition may also be particularly advantageous for hemiparasites. In xylem sap analyses, different host species have been reported to provide different resources to parasites (Govier et al. 1967; Seel and Jeschke 1999), and compared to grasses, legumes have been found to increase the nitrogen content and leaf chlorophyll content of attached parasites more strongly than their biomass, which is likely due to the legumes’ symbiosis with nitrogen-fixing rhizobia (Matthies 2017; Sandner and Matthies 2018). Complementarity in the resources obtained from grasses and legumes may explain why pot experiments with two or more different host species per parasite have often shown that a mixed diet of grasses and legumes is particularly beneficial for hemiparasites (Marvier 1998; Rowntree et al. 2014; Sandner and Matthies 2018, but see Matthies 1996). As Rhinanthus species naturally occur in grasslands, it may be beneficial for parasite seedlings to forage for legume roots, while grass roots are easily encountered by chance in the grassland matrix.
This is the first host-choice experiment for hemiparasites in an agar environment. The advantage of this approach compared to pot experiments (e.g. Sandner and Matthies 2018) is that the growth of the hemiparasite roots towards each host can directly be observed. However, the approach also has limitations. Most of all, the distance between host and parasite roots can hardly be standardized. Obligate parasites have to find host roots quickly after germination, and host tropism of the root tip has been found after only a few days in agar or on filter paper (Williams 1961; Whitney and Carsten 1981; Krupp et al. 2021). By contrast, many root hemiparasites like Rhinanthus species can grow autotrophically for weeks before attaching to suitable hosts (Oesau 1975; Heide-Jørgensen 2008), and host choice thus takes place at different spatial and temporal scales. Although we planted hosts at a standardized distance from the parasite and measured initial root length of the hosts to account for size differences, the host roots also increased their lateral extension. If parasite root growth stopped after reaching a host root, differences in host root growth could thus bias estimates of parasite lateral root growth. However, the lateral growth of parasite roots was not related to the lateral growth of host roots. Although some roots of the host species reached the other half of a Petri dish already after two weeks, the majority of the roots of each host were still in the half of the dish where the species had been planted. It is thus likely that parasite roots responded to a difference in the concentration of certain chemical compounds between the two halves of each experimental unit. We are confident that this method would be suitable for further studies on hemiparasite host choice.
To conclude, our results highlight the complexity of host choice and host quality in hemiparasitic Rhinanthus. The quality of plant species as a host depends on several unrelated processes including (1) the recognition of host roots leading to host tropism, (2) the successful formation of haustoria (which itself can be blocked at several stages, Yoshida and Shirasu 2009; Yoder and Scholes 2010), (3) the quality and quantity of resources obtained (e.g. Govier et al. 1967; Seel and Jeschke 1999) and (4) the competition with the host for resources (Matthies 1995). In each of these processes, hosts can be good or poor for hemiparasites. There may thus be no selection for directed growth towards ‘good hosts’. Instead, the observed differences in root growth towards different functional groups may be due to easier recognition of legumes (even legumes of poor quality as hosts) because of their chemical cues.
Data Availability
All data analysed for this study are included as a supplementary information file.
No funding was received for conducting this study, and the authors have no competing interests to declare that are relevant to the content of this article.
References
Albrecht H, Yoder JI, Phillips DA (1999) Flavonoids promote haustoria formation in the root parasite Triphysaria versicolor. Plant Physiol 119:585–592
Atsatt PR, Hearn TF, Nelson RL, Heineman RT (1978) Chemical induction and repression of haustoria in Orthocarpus purpurascens (Scrophulariaceae). Ann Bot (Oxford) 42:1177–1184
Atsatt PR (1977) The insect herbivore as a predictive model in parasitic seed plant biology. Amer Naturalist 111:579–586
Atsatt PR (1983) Host-parasite interactions in higher plants. In Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology III. Springer, Berlin, Heidelberg, pp 519–535
Berenbaum M (1983) Coumarins and caterpillars: A case for coevolution. Evolution 37:163
Cameron DD, Seel WE (2007) Functional anatomy of haustoria formed by Rhinanthus minor: linking evidence from histology and isotope tracing. New Phytol 174:412–419
Clarke CR, Timko MP, Yoder JI, Axtell MJ, Westwood JH (2019) Molecular dialog between parasitic plants and their hosts. Ann Rev Phytopathol 57:279–299
de Hullu E (1984) The distribution of Rhinanthus angustifolius in relation to host plant species. 3rd Int. Symp. on Parasitic Weeds. Aleppo, Syria, pp 43–52
Gibson CC, Watkinson AR (1989) The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia 78:401–406
Govier RN (1966) The inter-relationships of the hemiparasites and their hosts, with special reference to Odontites verna (Bell.) Dum. PhD thesis, University of Wales
Govier RN, Nelson MD, Pate JS (1967) Hemiparasitic nutrition in Angiosperms. I. The transfer of organic compounds from host to Odontites verna (Bell.) Dum. (Scrophulariaceae). New Phytol 66:285–297
Hartl D (1974) Scrophulariaceae, Rhinanthus. In Hegi G (ed.) Illustrierte Flora von Mitteleuropa. Carl Hanser Verlag, pp 374–403
Hautier Y, Hector A, Vojtech E, Purves D, Turnbull LA (2010) Modelling the growth of parasitic plants. J Ecol 98:857–866
Heide-Jørgensen HS (2008) Parasitic flowering plants. 1st ed., Brill, Boston
Holá E, Kocková J, Těšitel J (2017) DNA barcoding as a tool for identification of host association of root-hemiparasitic plants. Folia Geobot 52:227–238
Jiang F, Jeschke WD, Hartung W, Cameron DD (2010) Interactions between Rhinanthus minor and its hosts: A review of water, mineral nutrient and hormone flows and exchanges in the hemiparasitic association. Folia Geobot 45:369–385
Karban R (2021) Plant Communication. Ann Rev Ecol Evol Syst 52:1–24
Keith AM, Cameron DD, Seel WE (2004) Spatial interactions between the hemiparasitic angiosperm Rhinanthus minor and its host are species-specific. Funct Ecol 18:435–442
Krupp A, Bertsch B, Spring O (2021) Costunolide influences germ tube orientation in Sunflower Broomrape - a first step toward understanding chemotropism. Frontiers Pl Sci 12:699068
Kuijt J (1969) The biology of parasitic flowering plants, University of California Press, Berkeley
Marvier M (1998) A mixed diet improves performance and herbivore resistance of a parasitic plant. Ecology 79:1272–1280
Matthies D (1995) Parasitic and competitive interactions between the hemiparasites Rhinanthus serotinus and Odontites rubra and their host Medicago sativa. J Ecol 83:245
Matthies D (1996) Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: heterotrophic benefit and parasite mediated competition. Oikos 75:118
Matthies D (2017) Interactions between a root hemiparasite and 27 different hosts: growth, biomass allocation and plant architecture. Perspect Pl Ecol Evol Syst 24:118–137
Matthies D (2021) Closely related parasitic plants have similar host requirements and related effects on hosts. Ecol & Evol 11:12011–12024
Mescher MC, Smith J, Moraes CM de (2009) Host location and selection by holoparasitic plants. In Baluška F (ed.) Plant-environment interactions. Springer, Berlin, Heidelberg, pp 101–118
Mutuku JM, Cui S, Yoshida S, Shirasu K (2021) Orobanchaceae parasite-host interactions. New Phytol 230:46–59
Oesau A (1975) Untersuchungen zur Keimung und Entwicklung des Wurzelsystems in der Gattung Melampyrum L.(Scrophulariaceae). Beitr Biol Pflanzen 51:121–147
Oldroyd GED (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nature Rev Microbiol 11:252–263
Pate JS, Pate SR, Kuo J, Davidson NJ (1990) Growth, resource allocation and haustorial biology of the root hemiparasite Olax phyllanthi (Olacaceae). Ann Bot (Oxford) 65:437–449
Rehman F, Khan FA, Badruddin SMA (2012) Role of phenolics in plant defense against insect herbivory. In Srivastava MM, Khemani LD, Srivastava S (eds) Chemistry of phytopotentials: Health, energy and environmental perspectives. Springer, Berlin, Heidelberg, pp 309–313
Rowntree JK, Fisher Barham D, Stewart AJA, Hartley SE (2014) The effect of multiple host species on a keystone parasitic plant and its aphid herbivores. Funct Ecol 28:829–836
Rümer S, Cameron DD, Wacker R, Hartung W, Jiang F (2007) An anatomical study of the haustoria of Rhinanthus minor attached to roots of different hosts. Flora 202:194–200
Runyon JB, Mescher MC, Moraes CM de (2006) Volatile chemical cues guide host location and host selection by parasitic plants. Science 313:1964–1967
Sandner TM, Matthies D (2017) Interactions of inbreeding and stress by poor host quality in a root hemiparasite. Ann Bot (Oxford) 119:143–150
Sandner TM, Matthies D (2018) Multiple choice: hemiparasite performance in multi-species mixtures. Oikos 127:1291–1303
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Meth 9:671–675
Seel WE, Jeschke WD (1999) Simultaneous collection of xylem sap from Rhinanthus minor and the hosts Hordeum and Trifolium: hydraulic properties, xylem sap composition and effects of attachment. New Phytol 143:281–298
Serghini K, Pérez de Luque A, Castejón-Muñoz M, García-Torres L, Jorrín JV (2001) Sunflower (Helianthus annuus L.) response to broomrape (Orobanche cernua Loefl.) parasitism: induced synthesis and excretion of 7-hydroxylated simple coumarins. J Exp Bot 52:2227–2234
Sokal RR, Rohlf FJ (1995) Biometry. 3rd ed. W. H. Freeman and Company, New York
Suetsugu K, Kawakita A, Kato M (2008) Host range and selectivity of the hemiparasitic plant Thesium chinense (Santalaceae). Ann Bot (Oxford) 102:49–55
Tava A (2001) Coumarin-containing grass: Volatiles from Sweet Vernalgrass (Anthoxanthum odoratum L.). J Essential Oil Res 13:367–370
Těšitel J, Fibich P, Bello F de, Chytrý M, Lepš J (2015) Habitats and ecological niches of root-hemiparasitic plants: anassessment based on a large database of vegetation plots. Preslia:87–108
Těšitel J (2016) Functional biology of parasitic plants: a review. Pl Ecol Evol 149:5–20
Weber HC (1976) Über Wirtspflanzen und Parasitismus einiger mitteleuropäischer Rhinanthoideae (Scrophulariaceae). Pl Syst Evol 125:97–107
Whitney PJ, Carsten C (1981) Chemotropic response of Broomrape radicles to host root exudates. Ann Bot (Oxford) 48:919–921
Williams CN (1961) Tropism and morphogenesis of Striga seedlings in the host rhizosphere. Ann Bot (Oxford) 25:407–415
Yamamoto Y, Fujii Y (1997) Exudation of allelopathic compound from plant roots of Sweet Vernalgrass (Anthoxanthum odoratum). J Weed Sci Technol 42:31–35
Yoder JI (1997) A species-specific recognition system directs haustorium development in the parasitic plant Triphysaria (Scrophulariaceae). Planta 202:407–413
Yoder JI (2001) Host-plant recognition by parasitic Scrophulariaceae. Curr Opin Plant Biol, 4(4), 359–365
Yoder JI, Scholes JD (2010) Host plant resistance to parasitic weeds; recent progress and bottlenecks. Curr Opin Pl Biol 13:478–484
Yoshida S, Shirasu K (2009) Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytol 183:180–189
Acknowledgements
We thank Stephan Imhof and Belén Moncalvillo for help with the dissection and microscopy of the haustoria and for comments on an earlier draft of the manuscript. We also acknowledge comments by Renate Wesselingh and an anonymous reviewer which helped to improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sandner, T.M., Schoppan, L. & Matthies, D. Seedlings of a hemiparasite recognize legumes, but do not distinguish good from poor host species. Folia Geobot 57, 117–126 (2022). https://doi.org/10.1007/s12224-022-09414-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-022-09414-1