Abstract
Microbial natural products are among the main sources of compounds used in the medical biotechnology field for the purpose of drug development. However, as antibiotic resistance in pathogenic microorganisms is known to be increasing dramatically, there exists a need to develop new antibiotics. Actinomycetia have proven to be a good source of biologically active compounds, although the rediscovery of previously known compounds significantly slows down the introduction of new antibiotics. As a consequence, increasing attention is being paid to the isolation of actinomycete strains from previously unexplored sources, which can significantly increase the likelihood of discovering new biologically active compounds. This study investigated the diversity and bioactive potential of 372 actinomycete strains isolated from the rhizosphere soil of Juniperus excelsa M. Bieb. The examined actinomycete strains belonged to 11 genera, namely, Actinoplanes, Actinorectispora, Amycolatopsis, Kribbella, Micrococcus, Micromonospora, Nocardia, Promicromonospora, Rhodococcus, Saccharopolyspora and Streptomyces. The bioactive potential of each isolated actinomycete strain was determined on the basis of its ability to produce antimicrobial metabolites against Gram-positive and Gram-negative bacteria and yeast. Some 159 strains (42.74%) exhibited antimicrobial activity against at least one of the tested microbial strains. The dereplication analysis of the extract of the Streptomyces sp. Je 1–651 strain, which exhibited strong antimicrobial activity, led to the annotation of spiramycins and stambomycins. Moreover, the phylogenetic analysis based on the 16S rRNA gene sequence of the Je 1–651 strain revealed it to be close to the S. ambofaciens.
Similar content being viewed by others
Introduction
Natural products play a key role in drug discovery, especially when it comes to the treatment of infectious diseases and cancers (Newman and Cragg 2020). However, the rapid spread of bacterial infections has been observed in recent decades, mainly due to the rapid emergence and spread of multidrug-resistant (MDR) pathogens. One of the main reasons of this is the spread of MDR pathogens has demonstrated the lack of effective antibiotics to counteract and control them (Murray et al. 2022). As a consequence, there is now a general consensus that the discovery of new antibiotics would represent the best solution in the fight against antibiotic resistance among microorganisms.
The Actinomycetia are a class of bacteria known to be widely distributed in environments characterised by a high G + C content in the genomes. However, the main feature of the Actinomycetia that has attracted the attention of researchers is their ability to produce biologically active compounds, principally antibiotics. In fact, they produce more than 70% of all known antibiotics of natural origin, with the Streptomyces genus producing approximately 55% of all antibiotics (Demain and Adrio 2008; Hutchings et al. 2019). Unfortunately, following the ‘golden age’ of antibiotic discovery, which lasted from the 1940s to the 1960s, when the most common compounds were discovered and the producer strains were isolated, the discovery and clinical introduction of new antibiotics slowed considerably. The difficulty in introducing antibiotics can be explained by the significant rediscovery of previously known antibiotics, predominantly among the streptomycetes (Katz and Baltz 2016). One strategy with which to discover new bioactive compounds, including antibiotics, involves exploring new and understudied environments. Today, there are increasing reports of the discovery of new antibiotic producers in previously understudied environments. In particular, actinomycete strains from marine habitats (Jagannathan et al. 2021), deserts (Rateb et al. 2018), caves (Farda et al. 2022), Antarctica (Tistechok et al. 2021a) and other difficult to access or unexplored environments are being very actively studied. Moreover, the isolation of (rare) non-streptomycete genera from these environments increases the likelihood of discovering new biologically active compounds, as they are known to produce a number of antibiotics (Subramani and Sipkema 2019). Such studies confirm that much of the globe has been understudied in terms of antibiotic producer screening. Thus, continued research in under- and unexplored areas may lead to the discovery of important new antibiotics.
The diversity of the microbiome of the Crimean Peninsula, which is characterised by great variability with regard to its microclimatic conditions, has not been practically studied. Our previous studies have demonstrate successfully investigated certain streptomycete strains isolated from the rhizosphere of this peninsula in terms of their potential as producers of biologically active compounds (Tistechok et al. 2021b, 2022b), including previously unknown ones (Raju et al. 2013a, b, 2014, 2015). However, the diversity and biosynthetic potential of actinomycetes as producers of bioactive compounds from the rhizosphere soil of Juniperus excelsa M. Bieb. (also known as Greek juniper) in this region have not been described yet. Thus, in this study, we investigate the diversity of juniper rhizosphere actinomycetes and evaluate their potential for producing antimicrobial compounds.
Materials and methods
Actinomycete strains and growth conditions
The 372 actinomycete-like strains examined in this study were isolated in 2008 from rhizosphere soil of J. excelsa collected from Kischka Mountain on the Crimean Peninsula in Ukraine (Global Positioning System (GPS) coordinates: N 44° 24′ 02.07″ E 33° 59′ 32.96″). Three methods were used to isolate the actinomycetes, namely, (i) the direct inoculation of the aqueous-soil suspension, (ii) the pre-treatment of a soil sample with 1.5% aqueous phenol solution and (iii) the heating of the soil sample at 100 °C for 60 min. The obtained aqueous-soil suspension was plated on six different isolation media and then cultured for 14 days at 29 °C (Tistechok et al. 2018). The isolated strains were deposited in the Microbial Culture Collection of Antibiotic Producers of Ivan Franko National University of Lviv.
Oatmeal medium (OM) (Tistechok et al. 2022a) was used for the cultivation of the isolated strains. Liquid tryptic soy broth (TSB) (Sigma-Aldrich, St. Louis, MO, USA) was used for the cultivation of the actinomycete strains required for the total DNA extraction. In addition, SG medium (20 g/L glucose, 10 g/L soy peptone and 2 g/L CaCO3; pH 7.2) and DNPM medium (40 g/L dextrin, 7.5 g/L soy peptone, 5 g/L baker’s yeast and 21 g/L 3–[N-morpholino]propanesulfonic acid; pH 7.2) were used to produce the secondary metabolites.
Molecular identification of the actinomycete strains
The actinomycete-like strains for the total DNA extraction were cultivated in TSB medium for 3–5 days at a temperature of 28 °C and a shaking rate of 180 rpm. The total DNA extraction procedure utilised in this study has previously been described (Kieser et al. 2000).
The 16S rRNA gene was amplified by means of a polymerase chain reaction (PCR) using the universal primers 8F (5′-AGAGTTTGATYMTGGCTCAG-3′) and 1510R (5′-TACGGYTACCTTGTTACGACTT-3′). In a total volume of 50 μL, the PCR mixture consisted of 5 μL of 10 × PCR buffer, 1.0 μL of deoxynucleoside triphosphates (10.0 mmol/L each), 0.5 μL of each primer (100 pmol/L), 0.5 μL of DNA polymerase (1 U/μL), 2.5 μL of dimethyl sulfoxide, 2.0 μL of DNA template and 38.0 μL of Milli-Q-grade water. The PCR parameters were as follows: initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing of the primers at 53 °C for 30 s, extension at 72 °C for 90 s and a final extension at 72 °C for 10 min. The PCR products were visualised on 1% agarose gel. A QIAquick Gel Extraction Kit (Qiagen, Venlo, Netherlands) was used for the purification. The sequencing of the purified samples (5 μL) was performed by GENEWIZ from Azenta Life Sciences (Leipzig, Germany) in accordance with the manufacturer’s recommendations. The obtained 16S rRNA gene sequences were analysed using Geneious version 9.1.2 software (Kearse et al. 2012). The taxonomic affiliations of the isolated actinomycete strains were determined using Ribosomal Database Project Release 11 (https://rdp.cme.msu.edu/index.jsp). The 16S rRNA gene sequences of the isolated actinomycete strains were deposited in the National Center for Biotechnology Information (NCBI)’s GenBank (accession numbers available in Table S1).
A phylogenetic tree was constructed using the 16S rRNA gene sequences of the isolated actinomycete strains and their closest neighbours. The closest related species to the 16S rRNA were identified on the basis of the Basic Local Alignment Search Tool data available within the NCBI’s database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and then obtained from the GenBank. Moreover, the Multiple Sequence Comparison by Log-Expectation alignment tool was used to align the sequences (Edgar 2004). The phylogenetic tree was constructed based on this alignment using the neighbour-joining method with 1000 bootstraps according to the Kimura two-parameter method. This was performed using Molecular Evolutionary Genetics Analysis version 11 software (Tamura et al. 2021).
Screening for antimicrobial activity
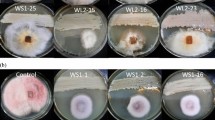
The primary screening for antimicrobial activity was performed for all of the isolated actinomycetes by means of the spot inoculation method (Tistechok et al. 2021a). More specifically, the actinomycete strains were spot inoculated on OM medium at seven strains per plate around the perimeter and then incubated at 28 °C for 6 days. The antimicrobial activity was tested against the following microorganisms: Staphylococcus aureus ATCC 25,923, Bacillus subtilis ATCC 31,324, Escherichia coli ATCC 25,922, Pseudomonas aeruginosa ATCC 9027, Klebsiella pneumonia ATCC 13,883, Proteus vulgaris ATCC 29,905 and Candida albicans ATCC 885–653. The bacterial strains were grown on Luria agar (10 g/L tryptone, 10 g/L NaCl, 5 g/L yeast extract and 15 g/L agar), whereas the yeast was grown on Sabouraud dextrose agar (Pronadisa (Conga), Madrid, Spain). The freshly grown test microbial strains were inoculated into 15 mL of the appropriate medium and then incubated in the proper conditions at a shaking rate of 180 rpm overnight. Next, the OM plates containing the 6-day-old actinomycete strains were covered with 5 mL of soft agar (0.7% w/v agar) that had previously been inoculated using one of the overnight cultures. The antimicrobial activity was observed on the basis of the appearance of zones revealing the growth inhibition of the test cultures. The antimicrobial activity index (AAI) was determined by measuring the ratio of the diameter of the inhibition zone to the diameter of the colony. The formula for determining the AAI was as follows:
Secondary metabolite extraction from the Streptomyces sp. Je 1–651 strain
For the secondary metabolite extraction, the Streptomyces sp. Je 1–651 strain was grown in 15 mL of TSB in a 100-mL flask for 2 days, before 1 mL of pre-culture was inoculated into 100 mL of production SG and DNPM media in a 500-mL flask. The Je 1–561 strain was grown for 7 days at 28 °C and 180 rpm in an Infors Multitron Incubation Shaker (Infors AG, Basel, Switzerland). The secondary metabolites of the Je 1–651 strain were extracted from the culture supernatant using an equal amount of ethyl acetate and acetone:methanol (1:1) mixture from the culture biomass. The obtained extracts were evaporated using an IKA RV-8 Rotary Evaporator (IKA, Staufen, Germany) at 40 °C and then dissolved in methanol.
Liquid chromatography–mass spectrometry (LC–MS) and dereplication analysis
The secondary metabolite extracts were analysed using a Dionex Ultimate 3000 UPLC System (Thermo Fisher Scientific, Waltham, MA, USA) coupled to a photodiode array (PDA) detector using a 100 mm ACQUITY UPLC BEH C18 1.7 μm column (Waters Corporation, Milford, MA, USA). A linear gradient (5 to 95%) of a water solution containing 0.1% (volume/volume (v/v)) formic acid (solvent A) and an acetonitrile solution containing 0.1% (v/v) formic acid (solvent B) as the mobile phase was used to separate the extracts at a flow rate of 0.6 mL/min for 18 min. The mass detection and analysis were performed with a Thermo LTQ Orbitrap XL (Thermo Fisher Scientific, Waltham, MA, USA) mass spectrometer using the positive mode of ionisation and a detection range of 200–2000 m/z. The gathered data were analysed using Thermo Xcalibur version 3.0 software. The monoisotopic masses were compared using the Dictionary of Natural Products (DNP) database (Buckingham 1993) with the following parameters: exact molecular mass, absorption spectrum, source of isolation, fragmentation and physical characteristics (Running 1993). Compounds were considered similar when the difference between their exact masses was less than 5 ppm and the absorption spectra were identical.
Results
Phylogenetic characterisation of the actinomycete strains
In our previous study, 372 actinomycete-like strains were isolated from the rhizosphere soil of J. excelsa. Most of the isolated strains exhibited the typical characteristics of actinomycetes, including slow growth, substrate and aerial mycelium formation, sporulation and pigment production. The taxonomic identification of these strains was evaluated on the basis of their 16S rRNA gene sequences (Table S1). The isolated actinomycete strains were distributed among seven families (Kribbellaceae, Micrococcaceae, Micromonosporaceae, Nocardiaceae, Promicromonosporaceae, Pseudonocardiaceae and Streptomycetaceae) and eleven genera (Actinoplanes, Actinorectispora, Amycolatopsis, Kribbella, Micrococcus, Micromonospora, Nocardia, Promicromonospora, Rhodococcus, Saccharopolyspora and Streptomyces). However, the majority of the isolates (350 or 94.08% of all the isolated strains) belonged to the genus Streptomyces.
The evolutionary relationships among the isolated actinomycete strains are demonstrated in the phylogenetic tree of the 16S rRNA gene presented in Fig. 1. All of the isolated strains were grouped within the respective genus and formed close clades with the 16S rRNA gene of representatives of the respective genera (Figs. S1–S6). Based on the evolutionary relationships of the 16S rRNA genes, the isolated strains of the genus Streptomyces were conditionally combined into seven groups. These groups formed clades consisting of both isolates and typical members of the genus Streptomyces and were named after them. Of the seven groups, the S. kanamyceticus group was the largest and included 103 isolated strains. There were also some strains among the isolates that were not combined into the formed groups. The non-streptomyce group included the group of isolates identified as non-streptomycetes and their closest neighbors, as shown in detail in Fig. S6.
Scheme of phylogenetic relationships of the actinomycete strains isolated from the rhizosphere soil of J. excelsa based on the 16S rRNA sequences. A phylogenetic tree was constructed by means of MEGA 11 using the neighbour-joining method with 1000 bootstrap replicates. The colours show the groups of isolates that formed common clades with typical members of the genus Streptomyces and were named after them. The 16S rRNA sequence of the E. coli strain U5/41 (NR_024570.1) was used as an outgroup
Analysis of the antimicrobial activity of the actinomycete strains
All of the isolated actinomycete strains were tested with regard to their ability to produce antimicrobial metabolites against Gram-positive bacteria, Gram-negative bacteria and yeast using the spot inoculation technique. Among the 372 isolated actinomycete strains, 159 strains (42.74%) exhibited antimicrobial activity against at least one of the tested microbial strains. Most of the strains inhibited the growth of Gram-positive bacteria, including B. subtilis ATCC 31,324 (132 strains, 35.48%) and S. aureus ATCC 25,923 (73 strains, 19.62%). However, significantly fewer strains inhibited the growth of Gram-negative bacteria. Here, 19 strains (5.11%) produced antimicrobial compounds against E. coli ATCC 25,922, whereas 14 strains (3.76%) and 18 strains (4.84%) were active against K. pneumoniae ATCC 13,883 and P. vulgaris ATCC 29,905, respectively, and only seven strains (1.88%) exhibited antagonistic activity against P. aeruginosa ATCC 9027. In addition, 36 strains (9.67%) exhibited antimicrobial activity against C. albicans ATCC 885–653.
Aside from ascertaining the ability of the isolated strains to inhibit the growth of a particular test culture, we also evaluated the level of antibiotic activity of these strains, which was calculated as the AAI. For this purpose, we grouped the different AAI into three categories: < 3 (low), 3–6 (medium) and > 6 (high). Most of the studied strains (from 1.08% of K. pneumoniae antagonists to 23.65% of B. subtilis antagonists) had a low AAI, whereas significantly fewer strains had a medium AAI (0.54–10.5%). The exception was the K. pneumoniae antagonists, among which 1.08% had an AAI < 3 while twice as many strains had a medium AAI. Only 2.71% of the strains had an AAI greater than 6, with no AAI greater than 6 being found for the E. coli, P. aeruginosa or C. albicans antagonists (Fig. 2).
Screening the antimicrobial activity of the actinomycete strains isolated from the rhizosphere soil of Juniperus excelsa. AAI Antimicrobial Activity Index, < 3 - grey, 3-6 light grey, > 6 - dark grey; Bs B. subtilis ATCC 31324, Sa S. aureus ATCC 25923; Ec E. coli ATCC 25922; Pa P. aeruginosa ATCC 9027, Kp K. pneumoniae ATCC 1388, Pv P. vulgaris ATCC 29905, Ca, C. albicans ATCC 29905
About half of the strains of the genus Streptomyces exhibited antimicrobial activity. Among the strains from other, less numerous genera, only a few showed antimicrobial activity. In particular, two representatives of the genus Amycolatopsis (strains Je 1–447 and Je 1–666) and the Actinorectispora sp. strain Je 1–571 suppressed the growth of the Gram-positive bacteria B. subtilis and S. aureus. Representatives of other genera of the isolated actinomycetes showed no inhibitory activity against the utilised test cultures.
Dereplication of the secondary metabolite profile of the Streptomyces sp. strain Je 1–651
After analysing the established collection of actinomycete strains, we identified several strains with a broad spectrum of antimicrobial activity, which captured both antibacterial and antifungal activities. In particular, the Streptomyces sp. Je 1–651 strain exhibited strong inhibitory activity (the AAI of this strain was at a medium level (3–6) or above) against all of the utilised microbial test cultures, except for P. aeruginosa. To better understand the nature of the compounds that may be responsible for the observed activity, we analysed the secondary metabolites produced by this strain. To accomplish this, we performed a dereplication analysis of the secondary metabolites of this strain within the DNP database (CRC Press). The ability of the strain Je 1–651 to produce secondary metabolites was studied by growing it in DNPM and SG liquid media. A total of 18 major secondary metabolite peaks were detected in the cultural liquid and biomass extracts of the Streptomyces sp. Je 1–651 cultivated in both media (Figs. 3, S7 and Table S2). Using the DNP database, seven of these peaks were identified as spiramycins. These peaks were present in all of the studied chromatograms, although their number and magnitude varied. In the crude biomass extract of the Je 1–651 strain, which was grown in SG medium, three large peaks were identified in addition to the spiramycins (Fig. 3b). These peaks were annotated as stambomycin A/B (retention time (tR) of 9.08; m/z 1376.9378 [M + H]+), stambomycin C/D (tR of 8.7; m/z 1362.9238 [M + H]+), stambomycin E (tR of 8.39; m/z 1348.9108 [M + H]+) and stambomycin F (tR of 9.66; m/z 1390.9558 [M + H]+) (Figs. 3b and S7).
LC–MS chromatogram of the ethyl acetate a and aceton:methanol b extracts of the Streptomyces sp. strain Je 1–651 after cultivation in SG (above) and DNPM (bottom) media. The medium peaks and identified antibiotic spiramycins and stambomycins are shown in grey. Red stars indicate compounds not identified in the DNP database
In the crude extracts of the Je 1–651 strain grown on DNPM medium, seven peaks were identified that did not yield positive matches in the DNP database and so could not be annotated based on the available mass spectrometry data. The absence of matches in the database may indicate the novelty of the associated compounds. The characteristics of the identified peaks, which likely form unknown compounds, are shown in Table S2.
Discussion
The rapid increase in the number of MDR pathogens seen in recent decades has stimulated the development of new therapeutic agents to combat them. Moreover, the urgent need for new antibiotics in a clinical setting has led to a revival of screening from nature. Microbial natural products are recognised as being among the most important elements when it comes to drug discovery (Pham et al. 2019). In this study, we analysed the phylogenetic diversity and antibiotic properties of actinomycete isolates from the rhizosphere soil of J. excelsa. The medicinal properties of J. excelsa are a reason for our interest in the rhizosphere actinomycetes of this species. The traditional Persian medicine used drugs based on these plants (Sargin et al. 2015). Extracts from different parts of this plant contain numerous metabolites that have antibacterial, antifungal, antiparasitic and other activities (Almaarri et al. 2010; Moein et al. 2017). Our preliminary studies of the metabolic potential of individual isolates of the juniper rhizosphere enabled us to identify several new compounds. In particular, leopolylic acid A (Raju et al. 2012b) which is considered as a potential protease inhibitor (Mpro) of SARS-CoV-2 (Mazzini et al. 2020), juniperolide A (Raju et al. 2012a) and furaquinocins K and L (Tistechok et al. 2022b). However, the phylogenetic diversity of actinomycetes of the Crimean Peninsula, in particular in the rhizosphere of J. excelsa, has not yet been described. Our taxonomic identification and phylogenetic analysis revealed among the assessed isolates representatives of seven families, including eleven genera of class Actinomycetia. Yet, Streptomyces was the most dominant genus among these isolates. This finding is unsurprising, as representatives of this genus are widely distributed in terrestrial ecosystems, especially in soil, where they play an important role in soil formation (Goodfellow and Williams 1983). Furthermore, as noted above, streptomycetes are a potential source of biologically active compounds, which may lead to further research into which new compounds may be identified.
We also isolated several non-streptomycete strains, among which we found representatives of some rather rare genera. In particular, we obtained three isolates (Je 1–106, Je 1–148 and Je 1–339) of the genus Promicromonospora (19 species have been identified to date (https://lpsn.dsmz.de/genus/Promicromonospora)) and strain Je 1–571 representing the genus Actinorectispora. This recently discovered genus of Actinomycetia currently has only two typical strains, namely, A. indica (Quadri et al. 2016) and A. metalli (Cao et al. 2018). Currently, there is no data on the range of secondary metabolites that can be produced by members of this genus. We also managed to identify members of the Amycolatopsis and Saccharopolyspora genera of the Pseudonocardiaceae, whose representatives are characterised by a great diversity of biosynthetic gene clusters and considered a promising source of new natural products (Gavriilidou et al. 2022). In future studies, a genome analysis of the actinomycete strains of these genera, as well as a chemical analysis of their metabolites, will allow us to reveal their biosynthetic potential. Furthermore, the presence of rare genera in the collection of strains isolated from the rhizosphere soil of J. excelsa, particularly the Actinorectispora genus, is promising and will contribute to further studies of the actinomycete diversity in this region.
The phylogenetic diversity of the actinomycete strains isolated from the rhizosphere of J. excelsa may be a guarantee of the rich composition of the associated biologically active compounds, among which there is a high probability of new compounds being discovered. One of the simplest approaches to evaluating a large collection of strains in terms of their ability to produce antimicrobial substances involves studying their antagonistic properties against specific test cultures (Balouiri et al. 2016). The actinomycete strains that we tested represented antagonists that specifically inhibited either Gram-positive (e.g. Je 1–101, Je 1–253 and others), Gram-negative bacteria (e.g. Je 1–208, Je 1–392 and others) or yeasts (e.g. Je 1–22.2, Je 1–93 and others). At the same time, some strains inhibited most or all of the utilised test cultures (e.g. Je 1–620, Je 1–651 and others) (Table S1). As previously noted, most of the strains that exhibited antibiotic activity were streptomycetes. Moreover, only some strains of the family Pseudonocardiaceae inhibited the growth of Gram-positive bacteria. Clearly, the non-streptomycete strains require more special fermentation conditions when compared with the streptomycetes (Amin et al. 2020). The wide spectrum of antimicrobial activity observed in this study indicates prerequisites for a deeper investigation of individual isolated strains.
As mentioned above, some of the studied strains demonstrated a wide range of antibiotic activity. Among them was the Je 1–651 strain, which exhibited the highest level of antimicrobial activity against the test cultures, except for P. aeruginosa. Additionally, we performed a dereplicative analysis of one promising strain that showed strong antimicrobial activity against the utilised test cultures. As a result, we were able to isolate the macrolide antibiotics spiramycin (Karray et al. 2007) and the 51-membered glycosylated macrolide stambomycin (Laureti et al. 2011). The dereplicative analysis allowed us to partially relate the observed biological activity of the Je 1–651 strain to specific compounds. We assume that the ability of the Streptomyces sp. Je 1–651 to inhibit the growth of Gram-positive bacteria, Gram-negative bacteria and yeast is due to its production of spiramycins, given their wide spectrum of activity (Labro 1993). It is also worth noting that spiramycins and stambomycins are produced by the S. ambofaciens strain (Aigle et al. 2013), which led us to assume that the Je 1–651 strain is perhaps phylogenetically related to the S. ambofaciens strains. A phylogenetic analysis based on the 16S rRNA gene sequence of the Je 1–651 strain showed similarity (100% identity) with type strains of S. ambofaciens. They also form a common clade on the phylogenetic tree (Fig. S8). Given the results of the phylogenetic analysis and secondary metabolite dereplication, we assume that Je 1–651 may be quite close to the S. ambofaciens strains. A detailed genomic analysis and comparison of these strains would improve our understanding of the similarities and differences between these strains. We do not exclude that the biosynthetic gene clusters of spiramycin and stambomycin may have differences, as was shown by the evolution of the antimycin biosynthetic gene cluster (Joynt and Seipke 2018). Such changes may provide selective advantages in the appropriate habitat. As the antibiotic spiramycin is in widespread use, further research on this strain is required, especially with respect to spiramycin production levels. In addition, the identification of new producers also has practical implications, as they may prove to be more convenient (e.g. fast growth, a higher production or are better suited for gene engineering manipulations) than currently utilised strains.
Furthermore, the use of different production media allowed us to examine the potential of the Je 1–651 strain in greater depth. Aside from spiramycin and stambomycin, several secondary metabolic peaks were detected in the extract of the Je 1–651 strain that we were unable to identify by means of the dereplicative analysis. This may indicate the potential novelty of these compounds, the structures of which we will try to determine in future studies.
Conclusions
In the present study, we demonstrated the diversity and bioactive potential of actinomycetes previously isolated from the rhizosphere soil of J. excelsa. Among the isolated strains, 11 genera were identified, including the quite rare genus Actinorectispora. Screening the antimicrobial activity of the isolated strains revealed their potential as producers of biologically active compounds. Moreover, the metabolic profiling of the widely active Streptomyces sp. Je 1–651 strain led to the identification of the commercially used antibiotic spiramycin as well as several potential new compounds. Thus, the findings of this study support the notion that actinomycete strains from unique ecosystems may be a potential source of producers of both pharmaceutical and biotechnological interests.
References
Aigle B, Lautru S, Spiteller D et al (2013) Genome mining of Streptomyces ambofaciens. J Ind Microbiol Biotechnol 41(2):251–263. https://doi.org/10.1007/s10295-013-1379-y
Almaarri K, Alamir L, Junaid Y, Xie D-Y (2010) Volatile compounds from leaf extracts of Juniperus excelsa growing in Syria via gas chromatography mass spectrometry. Anal Methods 2:673–677. https://doi.org/10.1039/b9ay00256a
Amin DH, Abdallah NA, Abolmaaty A et al (2020) Microbiological and molecular insights on rare Actinobacteria harboring bioactive prospective. Bull Natl Res Cent 44:5. https://doi.org/10.1186/s42269-019-0266-8
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Buckingham J (1993) Dictionary of natural products. CRC Press, London
Cao YR, He ZK, Guo Y et al (2018) Actinorectispora metalli sp. nov., a novel actinomycete isolated from a mine and emended description of the genus Actinorectispora. Int J Syst Evol Microbiol 68(4):1023–1027. https://doi.org/10.1099/ijsem.0.002620
Demain AL, Adrio JL (2008) Contributions of microorganisms to industrial biology. Mol Biotechnol 38(1):41–55. https://doi.org/10.1007/s12033-007-0035-z
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Farda B, Djebaili R, Vaccarelli I et al (2022) Actinomycetes from caves: an overview of their diversity, biotechnological properties, and insights for their use in soil environments. Microorganisms 10(2):453. https://doi.org/10.3390/microorganisms10020453
Gavriilidou A, Kautsar SA, Zaburannyi N et al (2022) Compendium of specialized metabolite biosynthetic diversity encoded in bacterial genomes. Nat Microbiol 7:726–735. https://doi.org/10.1038/s41564-022-01110-2
Goodfellow M, Williams S (1983) Ecology of actinomycetes. Ann Rev Microbiol 37:189–216
Hutchings MI, Truman AW, Wilkinson B (2019) Antibiotics: past, present and future. Curr Opin Microbiol 51:72–80. https://doi.org/10.1016/j.mib.2019.10.008
Jagannathan SV, Manemann EM, Rowe SE et al (2021) Marine actinomycetes, new sources of biotechnological products. Mar Drugs 19(7):365. https://doi.org/10.3390/md19070365
Joynt R, Seipke RF (2018) A phylogenetic and evolutionary analysis of antimycin biosynthesis. Microbiology (reading, England) 164(1):28–39. https://doi.org/10.1099/mic.0.000572
Karray F, Darbon E, Oestreicher N et al (2007) Organization of the biosynthetic gene cluster for the macrolide antibiotic spiramycin in Streptomyces ambofaciens. Microbiology (reading) 153(Pt 12):4111–4122. https://doi.org/10.1099/mic.0.2007/009746-0
Katz L, Baltz RH (2016) Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol 43(2–3):155–176. https://doi.org/10.1007/s10295-015-1723-5
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kieser B, Buttner M, Charter K, Hopwood B (2000) Practical Streptomyces genetics. John Innes Foundation, Norwich
Labro MT (1993) Pharmacology of spiramycin. Drug Invest 6(Suppl 1):15–28. https://doi.org/10.1007/BF03258433
Laureti L, Song L, Huang S et al (2011) Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens. Proc Natl Acad Sci USA 108(15):6258–6263. https://doi.org/10.1073/pnas.1019077108
Mazzini S, Musso L, Dallavalle S, Artali R (2020) Putative SARS-CoV-2 Mpro inhibitors from an in-house library of natural and nature-inspired products: a virtual screening and molecular docking study. Molecules 25(16):3745. https://doi.org/10.3390/molecules25163745
Moein M, Hatam G, Taghavi-Moghadam R, Zarshenas MM (2017) Antileishmanial activities of Greek juniper (Juniperus excelsa M.Bieb.) against Leishmania major promastigotes. J Evid Based Complementary Altern Med 22(1):31–36. https://doi.org/10.1177/2156587215623435
Murray CJL, Ikuta KS, Sharara F et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399:629–655. https://doi.org/10.1016/S0140-6736(21)02724-0
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83(3):770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Pham JV, Yilma MA, Feliz A et al (2019) A review of the microbial production of bioactive natural products and biologics. Fronti Microbiol 10:1404. https://doi.org/10.3389/fmicb.2019.01404
Quadri SR, Tian XP, Zhang J et al (2016) Actinorectispora indica gen. nov., sp. nov. isolated from soil, a member of the family Pseudonocardiaceae. Int J Syst Evol Microbiol 66(2):939–945. https://doi.org/10.1099/ijsem.0.000814
Raju R, Gromyko O, Andriy B et al (2014) Oleamycins A and B: new antibacterial cyclic hexadepsipeptides isolated from a terrestrial Streptomyces sp. J Antibiot (tokyo) 67(4):339–343. https://doi.org/10.1038/ja.2014.1
Raju R, Gromyko O, Fedorenko V et al (2012a) Juniperolide A: a new polyketide isolated from a terrestrial actinomycete. Streptomyces Sp Org Lett 14(23):5860–5863. https://doi.org/10.1021/ol302766z
Raju R, Gromyko O, Fedorenko V et al (2012b) Leopolic acid A, isolated from a terrestrial actinomycete, Streptomyces sp. Tetrahedron Lett 53:6300–6301. https://doi.org/10.1016/j.tetlet.2012.09.046
Raju R, Gromyko O, Fedorenko V et al (2013a) Oleaceran: a novel spiro[isobenzofuran-1,2’-naptho[1,8-bc]furan] isolated from a terrestrial Streptomyces sp. Org Lett 15(14):3487–3489. https://doi.org/10.1021/ol401490u
Raju R, Gromyko O, Fedorenko V et al (2013b) Rubimycinone A, a new anthraquinone from a terrestrial Streptomyces sp. Tetrahedron Lett 54(8):900–902. https://doi.org/10.1016/j.tetlet.2012.11.130
Raju R, Gromyko O, Fedorenko V et al (2015) Albaflavenol B, a new sesquiterpene isolated from the terrestrial actinomycete. Streptomyces Sp J Antibiot (tokyo) 68(4):286–288. https://doi.org/10.1038/ja.2014.138
Rateb ME, Ebel R, Jaspars M (2018) Natural product diversity of actinobacteria in the Atacama Desert. Antonie Van Leeuwenhoek 111(8):1467–1477. https://doi.org/10.1007/s10482-018-1030-z
Running W (1993) Computer software reviews. Chapman and hall dictionary of natural products on CD-ROM. J Chem Inf Model 33:934–935. https://doi.org/10.1021/ci00016a603
Sargin SA, Selvi S, Büyükcengiz M (2015) Ethnomedicinal plants of Aydıncık District of Mersin, Turkey. J Ethnopharmacol 174:200–216. https://doi.org/10.1016/j.jep.2015.08.008
Subramani R, Sipkema D (2019) Marine rare actinomycetes: a promising source of structurally diverse and unique novel natural products. Mar Drugs 17(5):249. https://doi.org/10.3390/md17050249
Tamura K, Stecher G, Kumar S (2021) MEGA 11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Tistechok S, Myronovskyi M, Fedorenko V et al (2022a) Screening of thiopeptide-producing streptomycetes isolated from the rhizosphere soil of Juniperus excelsa. Curr Microbiol 79:305. https://doi.org/10.1007/s00284-022-03004-2
Tistechok S, Skvortsova M, Mytsyk Y et al (2021a) The diversity and antibacterial activity of culturable actinobacteria isolated from the rhizosphere soil of Deschampsia antarctica (Galindez Island, Maritime Antarctic). Polar Biol 44(9):1859–1868. https://doi.org/10.1007/s00300-021-02924-2
Tistechok S, Stierhof M, Myronovskyi M et al (2022b) Furaquinocins K and L: novel naphthoquinone-based meroterpenoids from Streptomyces sp. Je 1–369. Antibiotics (Basel) 11(11):1587. https://doi.org/10.3390/antibiotics11111587
Tistechok SI, Syrvatka VJ, Fedorenko VO, Gromyko OM (2018) Actinomycetes of Juniperus excelsa Bield. rhizosphere – antagonists of phytopathogenic microbiota. Faktori Eksperimental’noi Evolucii Organizmiv 23:340–345 (in Ukrainian)
Tistechok SI, Tymchuk IV, Korniychuk OP et al (2021b) Genetic identification and antimicrobial activity of Streptomyces sp. strain Je 1–6 isolated from rhizosphere soil of Juniperus excelsa Bieb. Cytol Genet 55:28–35. https://doi.org/10.3103/S0095452721010138
Acknowledgements
This work was partially supported by the personal Grant 91657043 from the German Academic Exchange Service (DAAD) to ST.
Funding
This study was supported by grant H/309–2003 from Ministry of Education and Science of Ukraine.
Author information
Authors and Affiliations
Contributions
Conceptualization: ST, VF and OG; investigation: ST, IR and OG; writing—original draft preparation: ST; writing—review and editing: AL and OG; supervision: OG. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tistechok, S., Roman, I., Fedorenko, V. et al. Diversity and bioactive potential of Actinomycetia from the rhizosphere soil of Juniperus excelsa. Folia Microbiol 68, 645–653 (2023). https://doi.org/10.1007/s12223-023-01047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-023-01047-x