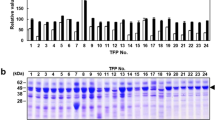
Abstract
During yeast dough fermentation, such as the high-sucrose bread-making process, the yeast cells are subjected to considerable osmotic stress, resulting in poor outcomes. Invertase is important for catalyzing the irreversible hydrolysis of sucrose to free glucose and fructose, and decreasing the catalytic activity of the invertase may reduce the glucose osmotic stress on the yeast. In this study, we performed structural design and site-directed mutagenesis (SDM) on the Saccharomyces cerevisiae invertase (ScInV) in an Escherichia coli expression system to study the catalytic activity of ScInV mutants in vitro. In addition, we generated the same mutation sites in the yeast endogenous genome and tested their invertase activity in yeast and dough fermentation ability. Our results indicated that appropriately reduced invertase activity of yeast ScInV can enhance dough fermentation activity under high-sucrose conditions by 52%. Our systems have greatly accelerated the engineering of yeast endogenous enzymes both in vitro and in yeast, and shed light on future metabolic engineering of yeast.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article with supplementary information files.
References
Álvaro-Benito M, Polo A, González B et al (2010) Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding. J Biol Chem 285:13930–13941
An Y, Ji J, Wu W et al (2005) A rapid and efficient method for multiple-site mutagenesis with a modified overlap extension PCR. Appl Microbiol Biot 68:774–778
Badotti F, Dário MG, Alves SL et al (2008) Switching the mode of sucrose utilization by Saccharomyces cerevisiae. Microb Cell Fact 7:1–11
Batista FR, Hernández L, Fernández JR et al (1999) Substitution of Asp-309 by Asn in the Arg-Asp-Pro (RDP) motif of Acetobacter diazotrophicus levansucrase affects sucrose hydrolysis, but not enzyme specificity. Biochem J 337:503–506
Fernandez-Moya R, Da Silva NA (2017) Engineering Saccharomyces cerevisiae for high-level synthesis of fatty acids and derived products. FEMS Yeast Res 17:fox071
Gomar-Alba M, Morcillo-Parra MÁ, del Olmo M, lí, (2015) Response of yeast cells to high glucose involves molecular and physiological differences when compared to other osmostress conditions. FEMS Yeast Res 15:1–14
Gong G, Zhang Y, Wang Z et al (2021) GTR 2.0: gRNA-tRNA array and Cas9-NG based genome disruption and single-nucleotide conversion in Saccharomyces cerevisiae. ACS Synth Biol 10:1328–1337
Hejlesen R, Füchtbauer EM (2020) Multiple site-directed mutagenesis via simple cloning by prolonged overlap extension. Biotechniques 68:345–348
Hernandez-Lopez MJ, Prieto JA, Randez-Gil F (2003) Osmotolerance and leavening ability in sweet and frozen sweet dough. Comparative analysis between Torulaspora delbrueckii and Saccharomyces cerevisiae baker’s yeast strains. Antonie Van Leeuwenhoek J G 84:125–134
Lagunas R (1993) Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol Lett 10:229–242
Liang X, Peng L, Li K et al (2012) A method for multi-site-directed mutagenesis based on homologous recombination. Anal Biochem 427:99–101
Lin X, Zhang CY, Bai XW, Xiao DG (2015) Enhanced leavening ability of baker’s yeast by overexpression of SNR84 with PGM2 deletion. J Ind Microbiol Biot 42:939–948
Lin X, Zhang CY, Meng L et al (2018) Overexpression of SNF4 and deletions of REG1- and REG2-enhanced maltose metabolism and leavening ability of baker’s yeast in lean dough. J Ind Microbiol Biot 45:827–838
Nagy ZB, Felföldi F, Tamás L, Puskás LG (2004) A one-tube, two-step polymerase chain reaction-based site-directed mutagenesis method with simple identification of the mutated product. Anal Biochem 324:301–303
Nielsen J, Larsson C, van Maris A, Pronk J (2013) Metabolic engineering of yeast for production of fuels and chemicals. Curr Opin Biotech 24:398–404
Ozcan S, Johnston M (1999) Function and regulation of yeast hexose transporters. Microbiol Mol Biol R 63:554–569
Peng RH, Xiong AS, Yao QH (2006) A direct and efficient PAGE-mediated overlap extension PCR method for gene multiple-site mutagenesis. Appl Microbiol Biot 73:234–240
Rabhi I, Guedel N, Chouk I et al (2004) A novel simple and rapid PCR-based site-directed mutagenesis method. Mol Biotechnol 26:27–34
Sainz-Polo MA, Ramírez-Escudero M, Lafraya A et al (2013) Three-dimensional structure of Saccharomyces invertase: role of a non-catalytic domain in oligomerization and substrate specificity. J Biol Chem 288:9755–9766
Sarokin L, Carlson M (1984) Upstream region required for regulated expression of the glucose-repressible SUC2 gene of Saccharomyces cerevisiae. Mol Cell Biol 4:2750–2757
Taussig R, Carlson M (1983) Nucleotide sequence of the yeast SUC2 gene for invertase. Nucleic Acids Res 11:1943–1954
Tokashiki T, Yamamoto H, Watanabe H et al (2011) A functional compound contained in sugar cane molasses enhances the fermentation ability of baker’s yeast in high-sugar dough. J Gen Appl Microbiol 57:303–307
Tomás-Cobos L, Casadomé L, Mas G et al (2004) Expression of the HXT1 low affinity glucose transporter requires the coordinated activities of the HOG and glucose signalling pathways. J Biol Chem 279:22010–22019
Varga-Orvos Z, Nagy ZB, Mészáros A et al (2007) Multiplex site-directed mutagenesis strategy including high-efficiency selection of the mutant PCR products. Biotechnol Lett 29:1921–1925
Voordeckers K, Brown CA, Vanneste K et al (2012) Reconstruction of Ancestral Metabolic Enzymes Reveals Molecular Mechanisms Underlying Evolutionary Innovation through Gene Duplication. PLoS Biol 10:e1001446
Zhang CY, Lin X, Feng B et al (2016) Enhanced leavening properties of baker’s yeast by reducing sucrase activity in sweet dough. Appl Microbiol Biot 100:6375–6383
Zhang Y, Nielsen J, Liu Z (2018) Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid-derived hydrocarbons. Biotechnol Bioeng 115:2139–2147
Zhang Y, Wang J, Wang Z et al (2019) A gRNA-tRNA array for CRISPR-Cas9 based rapid multiplexed genome editing in Saccharomyces cerevisiae. Nat Commun 10:1053
Zhao Y (2021) Production of β-carotene in Saccharomyces cerevisiae through altering yeast lipid metabolism. Biotechnol Bioeng 118:2043–2052
Zimmermann FK, Khan NA, Eaton NR (1973) Identification of new genes involved in disaccharide fermentation in yeast. Mol Gen Genet 123:29–41
Acknowledgements
We thanked Xintong Huang and Zan Chen from College of Veterinary Medicine, China Agricultural University, for assistance in quantifying invertase activity of ScInV mutants in vitro.
Funding
This work was supported by the National Natural Science Foundation of China (21908004 and 32100156), the Fundamental Research Funds for the Central Universities (buctrc201801), the Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Chinese Universities Scientific Fund, Outstanding Talent Introduction Program from College of Veterinary Medicine, China Agricultural University, Beijing Zhongnongda Veterinary Hospital Co. Ltd., and Shandong Bio Sunkeen Co. Ltd.
Author information
Authors and Affiliations
Contributions
LZ and ZYP supervised the study. ZYJ, MK, XS, LZ, and ZYP designed the experiments and analyzed the results. ZYJ and ZYP performed the experiments. MK, FJ, XS, and CG contributed reagents or analytical tools. ZY, MK, FJ, XS, CG, MD, JN, LZ, and ZYP all contributed to the overall study analysis, drafting, and reviewing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Appropriately reduced invertase activity of yeast ScInV point mutants (Q148A or Q201V) can improve dough fermentation activity under high-sucrose conditions.
• Our in vitro and in yeast site-directed mutagenesis systems can accelerate the engineering of yeast endogenous enzymes and could be a valuable tool to improve yeast characteristics.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Meng, K., Fu, J. et al. Protein engineering of invertase for enhancing yeast dough fermentation under high-sucrose conditions. Folia Microbiol 68, 207–217 (2023). https://doi.org/10.1007/s12223-022-01006-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-022-01006-y