Abstract
In modern textile-bleaching methods, H2O2 is commonly preferred due to its less harmful effects on the environment. The use of hydrogen peroxide in bleaching processes, although biodegradable on its own, increases the waste load due to the high amount of auxiliary chemicals used. The long processing times and high-temperature requirements of hydrogen peroxide bleaching led to increased energy consumption. Moreover, the high-water consumption required for post-treatment is also a disadvantageous factor. In other words, while the use of a high amount of auxiliary chemicals in hydrogen peroxide bleaching increases the waste load, long time and high-temperature requirements also increase energy consumption, and the high-water consumption required for post-treatment has a disadvantageous effect. In this study, raw cotton fabric is subjected to chemical finishing treatments that either oxidize or reduce it as part of the bleaching process. The results of photo bleaching were compared with conventional hydrogen peroxide finishing applications. Our findings showed that nano-TiO2-treated cotton had a better whiteness value than treated with H2O2 cotton according to the color spectrum whiteness indexes. It is strongly considered that this method could be a new alternative way for bleaching textile materials in the finishing departments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cotton fibers contain natural pigments that give it a light yellowish-brown color. In addition, dust, oil or human-induced contamination can also cause discoloration in the cotton fiber causes. The purpose of bleaching is to decompose these pigments and to ensure that the fibers gain a clean and white appearance. During bleaching, it is also ensured that the impurities that is swollen but unexfoliated during the hydrophilizing process, is removed. Bleaching of cotton fibers is generally done with oxidizing agents. To date, the cleanest and most used bleaching agent has been hydrogen peroxide among all bleaching agents due to its biodegradability properties. In modern textile-bleaching methods, the bleaching process, which is carried out by breaking down pigments to remove unwanted colors, is frequently performed with hydrogen peroxide. Hypochlorites have limited areas of use due to their environmental effects. However, environmental impact of hydrogen peroxide is quite high when it is considered in the area of textile auxiliary chemicals. In addition, hydrogen peroxide bleaching takes long time with high temperature and high amount of liquor which increase the energy and water consumption. Moreover, hydrogen peroxide bleaching is followed by post-processing/washing which also increased the amount of water used in whole finishing treatment [1,2,3,4,5,6]. If metals such as iron, copper, manganese and cobalt are present in the environment, H2O2 molecules begin to decompose very rapidly according to the radical chain mechanism and meanwhile oxidizes and damages cellulose molecules. For this reason, process parameters such as pH, temperature or heavy metal ions should be checked and kept under control during the bleaching process and if necessary anti-catalytic agents should be added into the finishing bath [7,8,9,10]. That is why it is considered that significant development and new bleaching methods/materials are required for removing target pigments that cause undesirable color on cotton or blended materials. Several research offer valuable information on sustainable bleaching techniques that are both efficient and environmentally beneficial [11, 12]. The utilization of natural zeolite in reducing hydrogen peroxide concentration can provide numerous benefits in textile-bleaching procedures [13]. One potential use for ozone, a potent oxidizing agent, is its bleaching effects in the textile industry [14]. The UV/H2O2 system provides a low-temperature method for both scouring and bleaching cotton fibers, resulting in an appealing white look [2].
Since it has a wide range of applications, great stability, minimal toxicity, and a low cost, titanium dioxide is the most popular and widely utilized photocatalyst [15]. Due to its relatively high surface oxygen density and low recombination of photogenerated electron–hole pairs, anatase, one of the three crystal forms of titanium dioxide (the others being rutile and brookite), is typically thought to have the highest photocatalytic activity [16]. For this reason, nano-anatase phase TiO2 has been chosen as the best candidate for photocatalytic reaction owing to the bandgap energy [17, 18]. On the surface of the material, electron and hole pairs are produced when energy-dense light is shone over the bandgap of nano-scale TiO2 [19]. Water can react with the oxidation-induced holes to produce hydroxyl radicals and electrons. The superoxide radical is reduced by oxygen, producing anions [20, 21]. As seen in the reaction depicted in Eq. (1), active oxygen species and hydroxyl radicals have the capacity to oxidize organic molecules into carbon dioxide and water [22]:
TiO2 photocatalysis has gotten a lot of attention because of its potential applications in anything from self-cleaning ability of teeth and self-cleaning cement to textile [23,24,25]. Due to the highly active radical species (superoxide ions and hydroxyl radicals are generated whenever photoelectrons from the TiO2 conduction band combine with molecules of oxygen, respectively) that are created at its surface under UV irradiation, TiO2 can take part in numerous oxidation reactions that destroy organic contaminants and ultimately lead to their mineralization [26, 27].
In recent years, there has been an increasing use of TiO2 in molecular form in textiles for various functionalities in the literature [22, 25, 28, 29]. However, there is a scarcity of studies addressing the formulation stages of TiO2 in the nano-size range. Simultaneously, very few studies have discussed the impact of this nano-sized material on bleaching and questioned its feasibility for industrial-scale application. Therefore, this article discusses a novel “nano photo bleaching” procedure for cleaning and bleaching desized cotton including optimization of finishing recipes. The fabrics that have been treated with nano-TiO2 particles on the surface also benefit from its self-cleaning property. The conventional bleaching process and the TiO2 nano-photocatalytic bleaching process were compared in terms of their whitening performance on raw cotton fabric. Chemical treatment recipe optimizations were performed to identify the most suitable finishing process for bleaching cotton fabrics, and all the characterization and performance results were evaluated in this study.
2 Materials and Methods
2.1 Materials
The materials for the bleaching studies were 100% woven cotton textiles (145 g/m2) made from Ne 20/1 fine yarns provided by Ege Özteks Tekstil, Uşak, Turkey.
2.2 Chemicals
Without additional purification, H2O2 (30%) and NaOH (48 Bé), were bought from Merck Co. Germany. Nanografi Co. Ltd. in Ankara, Turkey was the provider of the titanium dioxide (Purity: 99.55, anatase, 13 nm). The pre-treatment processes of cotton fabrics utilized alpha-amylase desizing enzyme and a wetting agent, all sourced from Alfa Kimya in Turkey.
2.3 Methods
During the pre-treatment, the desizing process was carried out using a Babkok pad-batch machine with an 80% wet pick-up ratio. This included the application of 2 g/L alpha-amylase desizing enzyme and 1 g/L wetting agent. Following this, 30 g/L NaOH (48 Bé) was applied to the cotton fabrics in the same machine to increase their hydrophilicity, in accordance with the industrial standards provided by Ege Özteks Tekstil. For the traditional bleaching method, a solution containing 30% (w/w) hydrogen peroxide (H2O2) at a concentration of 5 g/L (liquor ratio 1:10) was used.
Figure 1 shows an illustration of the photocatalytic experimental setup. Four 15 W UV–A lamps at a wavelength of 365 nm were utilized in the experimental configuration. The lamps were positioned in a symmetrical manner around the quartz tube within the galvanized cylinder along the reactor’s axis. This arrangement was done to enhance the yield and intensify the UV–A radiation. A beaker was first filled with the catalyst, and then 250 mL of distillated water was added to the beaker. An apparatus known as a stirrer was positioned beneath the photocatalytic system. After then, the catalyst and the fabric were rotated at a speed of 400 revolutions per minute for a period of 1 h. Experimental procedures were carried out in accordance with the parameters that are presented in Table 1, which details the experimental strategy. The UV intensity was measured using a meticulously calibrated UV radiometer (Labino, Apollo 3.0). The measured UV intensity of the photocatalytic device was 28.2 W/m2.
2.4 Characterization Tests of Nano-TiO2
X-ray diffraction (XRD) information collected on a diffractometer was used to establish the catalysts’ crystalline phase (Empyrean 2, Malvern Panalytical, Netherlands) using a Cu K radiation source in the range of 2 = 10—90° with a step size of 0.025° for TiO2. The surface was assessed with field emission scanning electron microscopy (XL-30 SFEG, Philips, Eindhoven, Holland). FTIR-ATR analysis (Perkin Elmer, Spectrum Two FT–IR) was performed to describe the functional groups of the nano-chemical.
2.5 Characterization and Performance Tests of Nano-TiO2-Treated and Untreated Cotton Fabrics
The examination of untreated and treated cotton samples involved a comprehensive approach, encompassing various characterization and performance tests. Utilizing a Philips XL-30 SFEG Scanning Electron Microscope (SEM) equipped with SEM–EDX for elemental analysis, cotton specimens coated with gold were meticulously imaged at 2000X magnification. Simultaneously, Fourier Transform Infrared Spectroscopy (FTIR) spectra were collected using a Spectrum Two FTIR spectrophotometer, providing insights into the molecular composition of the cotton samples. In addition to these characterization assessments, color spectrum and tearing strength tests were conducted to evaluate the color performance and mechanical strength of the cotton samples. Using a Konica Minolta CM-3600D Spectrophotometer from Japan, CIELAB color spectrum results, including L*, a*, b*, and ΔE (color changes between control and tested samples) values, were obtained. The tearing strength of the cotton samples was evaluated using the Elmendorf method, as outlined in ASTM D1424, for both the warp and weft directions. To determine the tear strengths of the fabrics, a D-type pendulum with a 64 N weight was employed.
3 Results and Discussion
3.1 Characterization Results of Catalyst
Figure 2 shows the FTIR spectrum of TiO2. The stretching vibrations of the hydroxyl group, which are both symmetric and asymmetric, are what cause the spectroscopic band to be seen at about 3260 cm−1 (Ti–OH)[30]. The adsorbed H2O molecule’s –OH bending vibration caused the other bending peak, which was seen at 1640 cm−1, to appear [31]. There are bands that correlate to the Ti–O–Ti stretch vibrations in the area between 800 and 400 cm−1, which is known as the “fingerprint gap” [32]. There is a very noticeable and wide TiO2 band in the 1000–600 cm−1 range [33]. At 792 cm−1, a TiO2 band was thus seen.
The surface morphology of the TiO2 nanoparticles was analyzed using scanning electron microscopy, as depicted in Fig. 3. The nanoparticles exhibit a spherical morphology with excellent dispersion. In addition, a decrease in the clustering of nanoparticles was observed, which could be attributed to the aggregation of primary TiO2 particles at elevated calcination temperatures, necessary for promoting the crystallization of titanium dioxide [34]. TiO2 surface morphology can be said to have a cauliflower-like shape [35]
Figure 4 shows the XRD spectrum of TiO2. The anatase phase of titanium dioxide’s signature peaks were found at 2θ = 25.31, 37.81, 48.05, 53.91, 55.06, and 62.68 [36], according to JCPDS card no. 21–1272 and Miller indices (101), (004), (200), (105), (211), and (204). Using the Bragg Law, it was found that TiO2 has d spacings of 0.349 nm. Scherer’s law determines the average crystal size of a substance. TiO2 was found to be 15 nm in size on average.
The UV–Vis spectral diffuse reflectance of TiO2 is displayed in Fig. 5. The considered TiO2 absorption tangent for anatase pattern was 378.70 nm. Using the Kubelka–Munk equation for an indirect allowed transition, the bandgap energy estimate for anatase TiO2 was found to be 3.11 eV. In the literature, there are calculations with similar outcomes [37].
3.2 Scanning Electron Microscopy SEM and SEM–EDX Characterization Results
The SEM images seen above clearly demonstrate the successful application of the photocatalytic bleaching method on 100% cotton fibers. In the initial image (Fig. 6a), the surface appears clean and smooth as no finishing process has been applied to the untreated cotton fibers. However, in all the images of photocatalytic processes carried out with TiO2 (Fig. 6b, Fig. 6c, Fig. 6d, Fig. 6e) to at different ratios and pH levels, as in Fig. 3, a cauliflower form similar to TiO2 is observed on the cotton fibers. In the final image (Fig. 6f), a significant etching effect of hydrogen peroxide application on the cotton fibers, which was performed under harsh conditions including high temperatures and high concentrations, could be seen clearly at the surfaces of the textile material. As a result of this surface characterization, it is concluded that the photocatalytic processes are chemically compatible with the SEM structure, since there is no chemical present on untreated cotton fibers, and regardless of the type and conditions of treatment, titanium dioxide particles are uniformly transferred through the photocatalytic process on the cotton fibers, whereas conventional hydrogen peroxide treatment leads to surface abrasion on the fibers. In support of the SEM analysis, SEM–EDX results (Fig. 7) show that in untreated cotton fiber (Fig. 7a), no elemental structure other than carbon and oxygen elements is detected. However, in the sample with the highest concentration (5 g/L TiO2), titanium element is also present at a weight ratio of 7.51% besides carbon and oxygen elements. In cotton fabric subjected to conventional hydrogen peroxide bleaching, the hydrogen molecule, because of being very small, could not be captured in the elemental analysis, but the presence of the other basic elements that make up the cotton fibers (carbon and oxygen) is demonstrated.
SEM images of a untreated cotton fabric, b 1 g/L TiO2 photocatalytic bleached fabric at pH 4.5 at 60 °C for 60 min, c 1 g/L TiO2 photocatalytic bleached fabric at pH 7 at 60 °C for 60 min, d 1 g/L TiO2 photocatalytic bleached fabric at pH 10.5 at 60 °C for 60 min, e 5 g/L TiO2 photocatalytic bleached fabric at pH 10.5 at 60 °C for 60 min, f conventional H2O2 bleached fabric
3.3 FTIR-ATR Analysis of Fabric Samples
The FTIR-ATR analysis results of the samples are depicted in Fig. 8. Notably, there were no significant peak disparities between the FTIR spectra of the untreated cotton fabric and the cotton fabric bleached with TiO2. These results underscore the resemblance in the spectral characteristics of TiO2 and cotton materials [38].The FTIR-ATR spectra of the TiO2-treated fabric and the untreated cotton fabric showed similarities because the TiO2 application did not generate distinct and characteristic peaks that could be associated with a different chemical compound. The natural FTIR spectrum of the cotton fabric already contains various organic components, but the TiO2 application did not significantly alter the fundamental characteristics of this spectrum. It was considered that as TiO2 is an inorganic compound compared to organic compounds like cellulose, it did not create distinct peaks in the FTIR spectrum.
As shown in Fig. 8, the OH functional groups present in cellulose manifest as distinctive bands at approximately 3300 cm–1. When the FTIR-ATR analysis of the untreated fabric was examined, distinctive peaks associated with cotton fibers were observed at the following wavenumbers: 3300 cm−1 (OH stretching), 1030 cm−1 (CO stretching), 2900 cm−1 (CH stretching), and 1310 cm−1 (CH vibration). The absorption bands at around 1300 cm−1 unveil the bending vibrations of the C–H and C–O groups in the cellulose polysaccharide rings. The intense peaks at 1030 cm−1 originate from the (C–O) and (O–H) stretching vibrations of polysaccharides within cellulose [39]. Furthermore, one dominant feature centered around 438 cm−1, which is characteristic of the TiO2 [40] and a vibration around 792 cm−1 which was a characteristic peak for anatase form of TiO2 [33], is illustrated in Fig. 8.
3.4 Color Spectrum Results
CIE L* a* b*, ΔE and whiteness index values were measured via spectrophotometer Konica Minolta CM-3600D, Japan. The results are shown in Table 2.
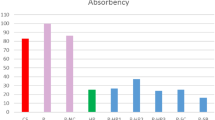
As seen in Table 2, when examining the color spectrum values, it is observed that the untreated fabric (S1) had a whiteness index of 63.15, while conventional bleaching process increased the whiteness index to 70.50, resulting in an 11.6% improvement. When the recipes formulated with titanium dioxide were evaluated independently, regardless of the pH range, the photocatalytic bleaching method performed the best results at 60 °C for 60 min. Photocatalytic bleaching carried out with very small amounts of 1 g/L of titanium dioxide resulted in a 14.2% increase in whiteness index at pH 4.5, reaching 72.12; 12.6% increase to 71.16 at pH 10.5, and at pH 7, the process yielded the highest value of 74.4, with a remarkable 17.8% increase. In experiments conducted at other temperatures (30 °C and 95 °C—conventional temperature), there was an increase in whiteness indices of up to 9%, but it was proven that these temperatures were not optimal for titanium dioxide photocatalytic bleaching. To evaluate the effect of the quantity, increase in photocatalytic bleaching, the amount was increased to 5 g per liter, and it was observed that the maximum whiteness index reached 72.62. This demonstrates that an increase in the quantity is not necessary when using titanium dioxide for photocatalytic bleaching, as whiteness indices do not significantly increase with higher quantities, contrary to this, the increase in quantity was unnecessary and even slightly altered the color values. Furthermore, when examining the CIE L*, a*, b* values as shown in Table 2, it was observed that there were no significant differences in ΔE values which identifies the differences between the final color values, between the treated and untreated cotton fabrics. This indicates that the optimal nano photo bleaching with TiO2 treatments did not significantly alter the visible color values and did not bring about a meaningful change in the fabric’s color which considered as a significant request and further advantage for sustainability in chemical finishing treatments. According to the results of this study in terms of color values, it is possible to achieve significantly better whitening performance with just 1 g/L of TiO2 at a low quantity and at lower temperature, consuming much less water and energy compared to conventional bleaching.
3.5 Tearing Strength Results
Figure 9 displays the tear strength measurements for all samples, both untreated and treated. The data from Fig. 9 reveal a reduction in tear strength in the warp and weft directions for all samples subjected to chemical or photocatalytic treatments. This reduction varied from about 13–17% in cotton fabrics treated with nano-TiO2 and was noted as 7.62% following conventional H2O2 treatment. The decrease in tear strength observed during conventional bleaching was attributed to the impact of hydrogen peroxide, which removes oil, wax, pectin, and natural ecru pigment from raw cotton, and its high oxidation capacity affecting the fibrils. This fibrillation was clearly demonstrated in the SEM images in Fig. 6, which corroborated the tear strength results. Furthermore, a more significant reduction in tear strength was observed in cotton samples treated with nano-TiO2. This could be due to an increase in the inter-fiber friction coefficient, as nano-scale chemicals penetrate deeper into the fibers’ surface area. In addition, several studies [41, 42] have indicated that TiO2 nanoparticles create a more rigid structure due to higher crosslinking, contributing to decreased tear strength. As inter-fiber friction increases, tear strength decreases, as shown in Fig. 9, which was in agreement with SEM images that show all nanoparticles existed between the fibers’ intersections. Nonetheless, the extensive penetration of nano-scale chemicals into the fibers and the photocatalytic process under UV exposure contributed to an overall decrease in tear strength, as seen in the SEM images in Fig. 4. In this study, aimed at industrial production, it was suggested that the observed decrease in tear strength could be mitigated using a softener to improve inter-fiber mobility.
4 Conclusion
In recent years, there has been a growing utilization of TiO2 in the textile industry for various purposes. However, there is a notable dearth of studies that delve into the intricacies of formulating TiO2 at the nano-scale. Moreover, there are few investigations that comprehensively assess the effects of this nano-sized material on the bleaching process while also scrutinizing its potential for large-scale industrial applications. Therefore, this article discusses a novel “nano photo bleaching” procedure for cleaning and bleaching desized cotton including optimization of finishing recipes. For this reason, nano-TiO2 in anatase form was used to perform finishing recipes and conventional H2O2 bleaching was compared to each other, in terms of color performances and characterization. According to the results of this study, it was determined that 1 g/L nano-TiO2, by displaying outstanding performance, reached the highest whiteness index level with the increase of 17.8% compared to untreated fabric and that notified even higher whiteness values when compared to the conventional bleaching on the cotton fabrics.
In conclusion, considering the high cost, machine corrosion, damage to fabrics, and the environmental burden associated with high-temperature conventional hydrogen peroxide bleaching, this study highlights the need for an alternative bleaching agent suitable for low temperatures and at low amount in the textile industry. The results of this study clearly indicate that working with 1 g/L of titanium dioxide within the appropriate pH range and temperatures results in significantly better whiteness than conventional bleaching, making it a more efficient process in terms of energy and water consumption. This study also considers that it can be easily scaled to industrial level by integrating UV lamps into finishing machines in the textile industry which can replace conventional applications.
Data Availability
Not applicable.
References
A. Farooq, S. Ali, N. Abbas, G.A. Fatima, M.A. Ashraf, Comparative performance evaluation of conventional bleaching and enzymatic bleaching with glucose oxidase on knitted cotton fabric. J. Clean. Prod. 42, 167–171 (2013)
N. Wang, P. Tang, C. Zhao, Z. Zhang, G. Sun, An environmentally friendly bleaching process for cotton fabrics: mechanism and application of UV/H2O2 system. Cellulose 27, 1071–1083 (2020). https://doi.org/10.1007/s10570-019-02812-3
K. Mojsov, Bioscouring and bleaching process of cotton fabrics–an opportunity of saving water and energy. J. Text. Inst. 107, 905–911 (2016)
C. Yin, Y. Huang, L. Zhang, H. Xu, Y. Zhong, Z. Mao, Low-temperature bleaching of cotton fabric using a copper-based catalyst for hydrogen peroxide. Color. Technol. 131, 66–71 (2015)
W. Du, D. Zuo, H. Gan, C. Yi, Comparative study on the effects of laser bleaching and conventional bleaching on the physical properties of indigo kapok/cotton denim fabrics. Appl. Sci. 9, 4662 (2019)
X. Fei, J. Yao, J. Du, C. Sun, Z. Xiang, C. Xu, Analysis of factors affecting the performance of activated peroxide systems on bleaching of cotton fabric. Cellulose 22, 1379–1388 (2015)
S.M. Shariful Islam, M. Alam, S. Akter, Identifying the values of whiteness index, strength and weight of cotton spandex woven fabric in peroxide bleaching of different concentration. Fibers Text. 26, 96–109 (2019)
J. Hayavadana, K. Samatha, Effect of copper sulphate on hydrogen peroxide bleaching with hydrolysis on P/C fabric mechanical properties. J Text. Eng Fash. Technol 2, 60 (2017)
X. Wang, J. Gao, Absorption spectra of gelatin copper complexes and copper (II) sulfate and their impact on cotton peroxide bleaching. J. Nat. Fibers 16, 307–318 (2019)
R.E. Brooks, S.B. Moore, Alkaline hydrogen peroxide bleaching of cellulose. Cellulose 7, 263–286 (2000)
S. Tripa, L. Indrie, P.D. Garcia, D. Mikucioniene, Solutions to reduce the environmental pressure exerted by technical textiles: a review o analiză a soluțiilor pentru reducerea presiunii exercitate de textilele tehnice asupra mediului. Ind. Textila. 75, 66–74 (2024)
M.T. Qamar, H.M. Mumtaz, M. Mohsin, H.N. Asghar, M. Iqbal, M. Nasir, Development of floating treatment wetlands with plant-bacteria partnership to clean textile bleaching effluent. Ind. Textila 70, 502–511 (2019)
C. Akca, A new method: the usage of natural zeolite as a killer chemical for hydrogen peroxide during the hydrogen peroxide bleaching. Ind. Textila 70, 519–522 (2019)
M.İ Bahtiyari, H. Benli, Comparison of ozone-based cold bleaching processes with conventional pretreatment of cotton. Ozone Sci. Eng. 42, 450–460 (2020)
Z.F. Yin, L. Wu, H.G. Yang, Y.H. Su, Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 15, 4844–4858 (2013)
S. Ardizzone, C.L. Bianchi, G. Cappelletti, S. Gialanella, C. Pirola, V. Ragaini, Tailored anatase/brookite nanocrystalline TiO2. the optimal particle features for liquid-and gas-phase photocatalytic reactions. J. Phys. Chem. C 111, 13222–13231 (2007)
H. Jiang, Q. Wang, S. Zang, J. Li, Q. Wang, Enhanced photoactivity of Sm, N, P-tridoped anatase-TiO2 nano-photocatalyst for 4-chlorophenol degradation under sunlight irradiation. J. Hazard. Mater. 261, 44–54 (2013)
P. Akhter, A. Arshad, A. Saleem, M. Hussain, Recent development in non-metal-doped titanium dioxide photocatalysts for different dyes degradation and the study of their strategic factors: a review. Catalysts 12, 1331 (2022)
Q. Xiang, J. Yu, M. Jaroniec, Enhanced photocatalytic H 2-production activity of graphene-modified titania nanosheets. Nanoscale 3, 3670–3678 (2011)
H. Chemingui, A. Moulahi, T. Missaoui, A.H. Al-Marri, A. Hafiane, (2022) A novel green preparation of zinc oxide nanoparticles with Hibiscus sabdariffa L.: photocatalytic performance, evaluation of antioxidant and antibacterial activity, Environ. Technol. 1–19.
M.V. Dozzi, L. Prati, P. Canton, E. Selli, Effects of gold nanoparticles deposition on the photocatalytic activity of titanium dioxide under visible light. Phys. Chem. Chem. Phys. 11, 7171–7180 (2009)
M. Montazer, S. Morshedi, Nano photo scouring and nano photo bleaching of raw cellulosic fabric using nano TiO2. Int. J. Biol. Macromol. 50, 1018–1025 (2012)
H. Kolsuz Ozcetin, D. Surmelioglu, Effects of bleaching gel containing TiO2 and chitosan on tooth surface roughness, microhardness and colour. Aust. Dent. J. 65, 269–277 (2020)
A. Saini, I. Arora, J.K. Ratan, Photo-induced hydrophilicity of microsized-TiO2 based self-cleaning cement. Mater. Lett. 260, 126888 (2020)
D. Selishchev, G. Stepanov, M. Sergeeva, M. Solovyeva, E. Zhuravlev, A. Komissarov, V. Richter, D. Kozlov, Inactivation and degradation of influenza a virus on the surface of photoactive self-cleaning cotton fabric functionalized with nanocrystalline TiO2. Catalysts 12, 1298 (2022)
R.B. Rajput, S.N. Jamble, R.B. Kale, A review on TiO2/SnO2 heterostructures as a photocatalyst for the degradation of dyes and organic pollutants. J. Environ. Manage. 307, 114533 (2022)
V. Augugliaro, M. Bellardita, V. Loddo, G. Palmisano, L. Palmisano, S. Yurdakal, Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 13, 224–245 (2012)
M.A. Barros, D.S. Conceição, C.G. Silva, M.J. Sampaio, J.L. Faria, Sustainable bleaching process of raw cotton by TiO2 light-activated nanoparticles. U. Porto J. Eng. 6, 11–21 (2020)
A. Farouk, S. Sharaf, M.M. Abd El-Hady, Preparation of multifunctional cationized cotton fabric based on TiO2 nanomaterials. Int. J. Biol. Macromol. 61, 230–237 (2013)
I.S. Elashmawi, A.A. Menazea, Dual laser ablation process assisted the synthesis of titanium dioxide and graphene oxide nanoparticles embedded in chitosan for electrical applications. Opt. Mater. 134, 113177 (2022)
G. Jnido, G. Ohms, W. Viöl, Deposition of TiO2 thin films on wood substrate by an air atmospheric pressure plasma jet. Coatings 9, 441 (2019)
S.S. Al-Taweel, H.R. Saud, New route for synthesis of pure anatase TiO2 nanoparticles via ultrasound-assisted sol-gel method. J. Chem. Pharm. Res. 8, 620–626 (2016)
Y. Zhang, A. Barber, J. Maxted, C. Lowe, R. Smith, T. Li, The depth profiling of TiO2 pigmented coil coatings using step scan phase modulation photoacoustic FTIR. Prog. Org. Coatings 76, 131–136 (2013)
Y.-F. Chen, C.-Y. Lee, M.-Y. Yeng, H.-T. Chiu, The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 247, 363–370 (2003)
L. Yang, Y. Lin, J. Jia, X. Xiao, X. Li, X. Zhou, Light harvesting enhancement for dye-sensitized solar cells by novel anode containing cauliflower-like TiO2 spheres. J. Power. Sources 182, 370–376 (2008)
X. Wei, G. Zhu, J. Fang, J. Chen, Synthesis, characterization, and photocatalysis of well-dispersible phase-pure anatase TiO2 nanoparticles. Int. J. Photoenergy 2013, 1–6 (2013)
J.-G. Li, T. Ishigaki, X. Sun, Anatase, brookite, and rutile nanocrystals via redox reactions under mild hydrothermal conditions: phase-selective synthesis and physicochemical properties. J. Phys. Chem. C 111, 4969–4976 (2007)
ŞS. Ugur, M. Sariişik, A.H. Aktaş, The fabrication of nanocomposite thin films with TiO2 nanoparticles by the layer-by-layer deposition method for multifunctional cotton fabrics. Nanotechnology 21, 325603 (2010)
Z.O. Basyigit, D. Kut, P. Hauser, Development of multifunctional cotton fabric via chemical foam application method. Text. Res. J. 90, 991–1001 (2020)
J.-Y. Zhang, I.W. Boyd, B.J. O’sullivan, P.K. Hurley, P.V. Kelly, J.-P. Senateur, Nanocrystalline TiO2 films studied by optical, XRD and FTIR spectroscopy. J. Non Cryst. Solids 303, 134–138 (2002)
S. Riaz, M. Ashraf, T. Hussain, M.T. Hussain, A. Younus, M. Raza, A. Nosheen, Selection and optimization of silane coupling agents to develop durable functional cotton fabrics using TiO2 nanoparticles. Fibers Polym. 22, 109–122 (2021)
G. Gedik, Bleaching of cotton/lyocell fabrics with heterogeneous photocatalysis with titanium (IV) oxide under UV light and investigation of the effect of oxygen radicals on bleaching process. Tekst. ve Muhendis 27. 118, 64–74 (2020)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). No organizations provided funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent to Participate
This article does not contain any studies with human participants or animal performed by any of the authors.
Consent for Publication
All the authors have provided their consent for publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basyigit, Z.O., Ciğeroğlu, Z. Nano Photo Bleaching Method of Cotton Fabrics for a Sustainable Finishing. Fibers Polym (2024). https://doi.org/10.1007/s12221-024-00642-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12221-024-00642-0