Abstract
The goal of this study is to present an environmentally benign route for cotton fabric and investigate the effect of different pretreatments in natural dyeing. In this context, conventional alkaline scouring, conventional hydrogen peroxide bleaching, low-temperature one-step biopreparation processes, namely enzymatic scouring (alkaline pectinase and alkaline pectinase-neutral cellulase combination) at 55 °C and enzyme-bleaching agent (hydrogen peroxide/sodium percarbonate/sodium perborate)-activator agent (TAED) combinations at 65 °C were applied to 100% cotton knitted fabric. The use of agricultural waste and eco-friendly mordants was preferred in natural dyeing. For this purpose, pretreated fabrics were dyed with the outer green shell of almond fruit extracts and a low amount of 0.4 g/L metal mordants (alum and iron(II) sulfate) in accordance with the simultaneous mordanting method. The dyeing properties of bio- and conventionally prepared cotton fabrics were examined in terms of colorimetric data (K/S, CIELa*b*C*h°) and wash fastness compared with water absorbency, whiteness, weight loss, pectin removal, and type of mordant. Excellent wash fastness values were achieved regardless of the type of pretreatment. Low-temperature one-step biopreparation can be a good substitute for conventional scouring and bleaching processes. Since different results can be achieved, it is essential to determine and evaluate all bioprocess conditions depending on the end-use characteristics of the textile (e.g. whether it will be white or dyed/printed, its color and lightness/darkness) at the laboratory and industrial scale applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Environmental regulations and the growing need for sustainable and eco-friendly textile wet processes force researchers to develop cost-effective, less water- and energy-consuming novel alternatives to conventional pretreatments. Conventional scouring and bleaching processes have huge environmental impacts due to the use of harsh chemicals and large amounts of water and energy. Enzymes, peroxide bleach activators, some auxiliaries, and novel agents can be used to reduce the time and temperature of textile pretreatments. Less water- and energy-consuming biopreparation processes such as enzymatic scouring and bleaching are considered ecofriendly alternatives to conventional pretreatments.
Scouring is an important wet process in which non-cellulosic impurities of cotton are removed to purify the fibers. It is possible to perform the scouring process using sodium hydroxide, enzymes, or solvent extraction [1]. Scouring and bleaching improve water wettability and water retention. Bleaching does not change the fabric pore structure while scouring reduces the pore volume [2]. Researchers investigated the effect of various enzymes and biopretratmens on cotton structure versus conventional scouring and bleaching processes. Enzymatic scourings [3,4,5,6,7,8,9,10], combined alkaline scouring/bleaching and enzymatic scouring/bleaching [11], alkaline pectinase-oxidative agent-activator agent combination [12], H2O2 /TAED and H2O2/TBCC bleaching [13] using mild working conditions provided satisfactory results as an alternative to conventional scouring in terms of absorbency, whiteness, color yield, and strength loss. After enzimatic pretreatments, dyeing and water absorbency properties of fabrics are essential for satisfactory dyeing/printing results [3]. Tetraacetylethylenediamine (TAED) which is an eco-friendly bleaching activator improves fabric bleaching performance at lower temperatures [14,15,16,17,18,19,20] and activates bleaching reactions at low temperatures and in a milder pH medium. It is the most commonly used oxygen activator in bleaching systems [21, 22]. It reacts with hydrogen peroxide to generate peracetic acid under alkaline conditions. Peracetic acid has a higher oxidation potential (1.81 eV) than hydrogen peroxide (1.33 eV) [23]. TAED-activated peroxide system has the potential for bleaching cotton with improved bleaching effectiveness under mild conditions. It is a white solid organic compound that is prepared by acetylating ethylenediamine. One mole of TAED reacts with two moles of the perhydroxide anion to form two moles of the peracetic anion and one mole of diacetyl ethylene diamine (DAED). Both TAED and the reaction product DAED are nontoxic, non-sensitizing, and biodegradable by microorganisms into carbon dioxide, water, ammonia, and nitrates [23,24,25,26,27]. TAED is also used to activate sodium percarbonate and sodium perborate for cotton bleaching [28, 29]. Hydrogen peroxide, sodium percarbonate, sodium perborate, and sodium persulfate, were activated with TAED to degrade a reactive azo dye present a novel and rapid oxidative system [30]. One-bath enzyme scouring and percarbonate bleaching were also used for the enzymatic pretreatment of wool. Higher absorbency, color yield and whiteness, less felting without negative morphological changes or chemical damage to the fibers [31]. The outer green shell of almond fruit, which is a huge amount of agricultural waste producing a wide and rich color gamut, was used for the first time [32] and further investigated using eco-friendly methods such as US, plasma, and biomordants in the natural dyeing of wool [33,34,35]. In this study, the natural dyeing properties of bio- and conventionally prepared cotton fabric with the outer green shell of an almond were examined in terms of color strength, color coordinates, and washing fastness depending on the type of mordant, water absorbency, pectin removal, weight loss and whiteness measurements.
2 Experimental
2.1 Material
Single jersey 100% cotton knitted fabric (135 g/m2) was used in the study.
2.2 Pretreatments
Conventional alkaline scouring, enzymatic scouring, hydrogen peroxide bleaching, and low-temperature one-step bioscouring and bleaching combinations (Table 1) were conducted in Ataç IR laboratory dyeing machine (Türkiye) at a liquor ratio of 50:1. Alkaline pectinase (Novozymes, Bacillus microorganism with 3000 APSU/g activity) and neutral cellulase (Novozymes, Humicola microorganism with 1100/g DAU activity), kindly supplied by Novozymes, were used in enzymatic scouring. The recipes were arranged based on the reference [12].
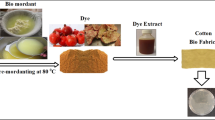
2.3 Extraction of the Natural Dye
Outer green shells of almond fruit were dried and ground to use in natural dyeings. The powder (1 g) was extracted by boiling in water (150 mL) for 1 h. The extracts were filtered to remove insoluble residues, and the reduced part of the extract during boiling was brought up to 150 mL with water.
2.4 Dyeing
Fabric samples (3 g) were dyed with extracted dye solution by the exhaust method (50:1 liquor ratio) according to the simultaneous mordanting method using 0.4 g/L of alum (dodecahydrate) (potassium aluminum sulfate) and 0.4 g/L of iron (II) sulfate (heptahydrate) in Ataç IR laboratory dyeing machine (Türkiye). The dye bath temperature was gradually raised (at about 1 °C/min) to 100 °C and maintained at this temperature for 60 min. The dyed samples were rinsed thoroughly with water and dried at room temperature.
2.5 Testing
Water absorbency values of fabrics were determined according to the AATCC 79 water drop test method. The weight loss was calculated according to Eq. (1).
Pretreated samples were dyed in a bath containing 0.2 g/L Ruthenium Red at room temperature for 15 min and subsequent rinsing, washing at 60 °C for 5 min, air drying at room temperature. K/S values were measured to evaluate pectin removal. The higher the amount of Ruthenium Red on the fabric, the higher the pectin content. Whiteness (Stensby) and color measurements were made using a HunterLab UltraScan PRO spectrophotometer with illuminant D65 and the CIE 10° observer at the individual wavelengths of maximum absorption. The color yield (K/S) was calculated according to the Kubelka–Munk equation.
where K is the coefficient of absorption, S is the coefficient of scattering, and R is the reflectance at the maximum absorbance wavelength. Washing fastness of the dyed samples was tested using ISO105 C06 test method (at 40 °C for 30 min).
3 Results and Discussion
3.1 Water Absorbency
Water absorbency is one of the most important goals of cotton pretreatment processes. Successful and satisfying dyeing/printing and finishing results involve homogenous and consistent absorbency. It is a property that is affected by various factors such as the composition of the material, amount of impurities, yarn and surface properties, moisture, and thickness.
Absorbency properties are shown in Fig. 1. The higher the value of the fabric, the lower the absorbency in the water drop test method. Although there were no significant differences, conventional scouring had better absorbency than enzymatic scouring. Alkaline pectinase and neutral cellulase combination enhanced the absorbency due to the synergistic effect of the enzymes. There was a remarkable increase in the absorbency values of conventional bleaching and enzyme-bleaching combinations. In all samples, the enzyme–sodium porborate-TAED combination provided the best and most different absorbency from the others.
Enzymatic and only buffer treatment with a suitable surfactant improves the absorbency of cotton for dyeing, printing, and finishing. Water absorbency obtained with or without enzyme is sufficient and equal to that of conventional scouring [3]. However, absorbency alone is not sufficient for satisfactory dyeing results. Although homogeneous and good absorbency values are prerequisites for successful dyeing/printing, it is also necessary to pay attention to the changes in the fiber structure caused by the pretreatment. These structural changes and porosity have important effects on the dyeing results obtained.
Pore structure of the fiber affects water retention and absorbency [2]. Alkaline scouring improves the wetting properties but lowers the pore volume for water retention. Although water retention reduced, the overall water retention increased due to enhanced water absorbency. Bleaching improves surface wettability without changing the fabric pore structure. Improved surface wettability increases water retention [2]. Absorbency changed depending on the type of enzymes and a high degree of positive correlation was observed between wax and pectin content. The sample with relatively high pectin or wax content can be satisfactorily absorbent. Wax content was the most effective factor in absorbency. This was followed by pectin content and weight loss, respectively. Wax content was more effective than pectin content in weight loss [1].
3.2 Whiteness
Figure 2 shows the relative (%) whiteness of differently pretreated fabrics. Conventional scouring ensured better whiteness than enzymatic scouring. The whiteness of conventional bleaching was significantly higher than that of conventional scouring. Whiteness of the enzyme combination was slightly higher than that of alkaline pectinase. The relative whiteness of enzymatic scourings (75.7 and 76.7%) was very near and significantly lower than that of the conventional bleaching. The whiteness values of enzyme-sodium percarbonate-TAED (P-SC) and enzyme-sodium perborate-TAED (P-SB) were the same and lower than those of the enzyme-hydrogen peroxide combinations. There was no significant difference between the whiteness degrees of enzyme-hydrogen peoxide-TAED combinations. However, enzyme-hydrogen peroxide—4 g/L TAED (P-HP3) had the closest whiteness to conventional bleaching. Whiteness increased as TAED concentration increased in enzyme-peroxide bleaching combinations. P-HP3 had the highest whiteness, whereas sodium percarbonate (P-SC) and sodium perborate (P-SB) had the lowest whiteness.
3.3 Weight Loss
Figure 3 shows the relative (%) weight loss values of bio- and conventionally pretreated fabrics. The weight loss values of conventional scouring and bleaching are almost the same. Conventional scouring generated the highest weight loss, followed by conventional bleaching. Alkaline pectinase had the lowest weight loss. It was observed that the combination of pectinase and cellulase produced higher weight loss than pectinase scouring. Bioscoured samples have an advantage over conventional scouring and bleaching in terms of weight loss. Enzymatic scouring caused lower weight loss than conventional scouring and hydrogen peroxide bleaching. There were not much weight loss differences between the enzyme-bleaching-TAED combinations. Among enzyme and bleaching combinations, hydrogen peroxide with 2 g/L TAED (P-HP2) had the highest (75.5%) and sodium perborate (P-SP) had the lowest (73.1%) weight loss. Enzyme-sodium percarbonate (P-SC) and enzyme-sodium perborate (P-SB) combinations with very close values produced the lowest weight losses. However, no significant differences were detected between the enzyme-bleaching agent-TAED combinations in terms of weight loss. The type of bleaching agent and TAED concentration had no significant effect on weight loss.
3.4 Pectin Removal
Relative (%) pectin removal is shown in Fig. 4. The higher (K/S) the amount of Ruthenium Red dye on the fabric, the higher the content of pectinic substances and proteins on the surface of cotton fabrics. Considering the K/S values of Ruthenium Red dyeing, it is seen that all enzymatic processes exhibited better pectin removal than conventional scouring and bleaching. This finding shows the positive effect of alkaline pectinase on pectin removal. Usually, absorbency increased as the pectin content decreased. Among all samples, conventional bleaching had the worst pectin removal, whereas the combination of pectinase and cellulase was the best. Among the enzyme-bleaching agent-TAED combinations, enzyme-hydrogen peroxide combinations provided the best pectin removal. Although there was no significant difference between enzyme-sodium perborate and enzyme-sodium percarbonate combinations, they provided a lower pectin removal effect than hydrogen peroxide.
3.5 Effect of Pretreatment on Color Yield (K/S) in Natural Dyeing with Iron Mordant and the Relationship Among Absorbency, Whiteness, Weight Loss, Pectin Removal, and Color Yield
The relative color yield with iron mordant is demonstrated in Fig. 5. Color darkness is effective in making enzymatic biopreparations a viable replacement for conventional processes. In previous studies, biopretreated cotton fabrics were homogeneously dyed with reactive dyes equal to conventional scouring. Reactive dyeing resulted in fabrics with a color evenness equal to that of alkaline-scoured and dyed fabric. However, dye concentration was effective on different dyeing results. Higher color differences at lower dye concentrations (0.2 and 0.5%) and no perceptible color differences at higher dye concentrations (1 and 2%) occurred between biopretreated and conventionally scoured samples [3, 12, 36]. No significant difference was observed between untreated and enzymatically treated cotton in Direct dyeing. Enzymatic treatment did not cause a remarkable increase in color strength [37,38,39]. Biopolishing of cotton fabric can play an important role in changing the dyeability of fabric. It depends whether the cellulose treatment is performed before or after dyeing. As no change in the crystallinity is observed due to cellulase pre-treatment, no change in the dyeability of cellulose has been reported [40]. However, in this study, cellulase was used along with alkaline pectinase, and this synergistic effect increased the color strength. There are conflicting studies regarding the effect of enzymatic pretreatments on dyeing. It is thought that the type of dye, enzyme, process, fiber, and even the source of enzyme have important effects on these different results.
Significant color differences were revealed between the biopretreated and alkaline scoured fabrics due to the much cleaner and whiter surface of the conventionally scoured fabric. However, hydrogen peroxide bleaching after biopretreatment significantly reduced color differences [3]. It has been reported that color yield (K/S) improved with enzymatic pretreatment [12, 36, 41, 42]. In this study, the pectinase–cellulase combination (P-NC) provided a significantly higher color yield than alkaline pectinase alone and the closest color yield to that of conventional alkaline scouring. Although the color yield of the conventional alkaline scouring could not be fully reached by enzyme combination, the difference between them was imperceptible. The color yield of the enzyme combination was higher than that of conventional bleaching and enzyme-bleaching agent-TAED combinations, except for the enzyme-hydrogen peroxide-1 g/L TAED combination (P-HP1). In the case of enzyme-bleaching-TAED combinations, the enzyme increased the color yield compared with conventional bleaching. The enzyme-hydrogen peroxide combination with 1 g/L TAED (P-HP1) had the best color yield of all samples, followed by conventional scouring and then the pectinase-cellulase combination. The type of dye and the process are effective factors, and quite different results can be obtained accordingly.
Cellulase increases the color yield by changing and making more accessible the structure of cotton. Its biopolishing effect is also important for color measurements [43]. The removal of protruding fibers decreases the scattering coefficient, which depends on the degree of polymerization, ratio of amorphous to crystalline regions, swellability, accessibility, chemical reactivity, surface morphology, and affinity of dyes [41, 42]. The pore volume and surface area of cotton are essential factors in terms of the accessibility of dyes and chemicals. Cellulase changes the pore volume and surface area of cotton through hydrolysis of cellulose. The volume and surface area of pores smaller than 60 A° decrease after cellulase treatment and does not change for pores larger than 60 A°. Consequently, small pores significantly decrease [37]. Cellulase is very large compared with the pores in cotton and can access only 6.7% of the pore volume and 1.1% of the surface area. While the porosity change due to enzyme treatment is small, the weight loss and the breaking strength loss are relatively large [37]. Cellulase reduces the problems regarding poor dyeing properties of immature fiber neps [44]. The literature concerning the structural effect of cellulase supports the findings of dyeing, weight loss, and pectin removal results of this study. Duration and temperature are also essential factors in biopreparation. It has been reported that 30 min of biotreatment favored the maximum color strength of reactive dyes. Longer times reduced the color yield. The highest color yield was achieved upon increasing the biotreatment temperature from 20 to 40 °C [44].
The relative absorbency and color yield are shown in Fig. 6. The enzyme-sodium porborate-TAED combination had the best absorbency but the lowest color yield. No general conclusion can be reached that the sample with the best absorbency yields the highest color strength or that the samples with the same/near absorbency values result in similar color yields. For example, while conventional bleaching (HP) and the enzyme-sodium percarbonate-TAED combination (P-SC) had the same absorbency, their color yields were 82 and 85%, respectively. The enzyme-sodium perborate-TAED combination (P-SB) with the best absorbency had the lowest (71%) color yield. Although the absorbency of sodium perborate was significantly better than that of sodium percarbonate, its color yield was significantly lower. This finding suggests that absorbency is not the only factor affecting color yield.
The relative whiteness and color yield with iron mordant are shown in Fig. 7.
If the whiteness of the fabric is higher, the color may appear lighter; however, this is not a strict rule. Although the whiteness of the pectinase–cellulase enzyme combination (P-NC) was slightly higher than that of pectinase (P), its color yield was 90%, whereas alkaline pectinase alone had a lower color yield (72%). Similarly, although the whiteness of conventional bleaching (100%) was much higher than that of alkaline pectinase (75.7%), its color yield was higher than that of pectinase. However, the color yield of pectinase was lower than that of the combination of pectinase and cellulase. The whiteness of conventional bleaching is significantly higher than that of conventional alkaline scouring, but the color yield is lower. Alkaline scouring had a better color yield. There was a significant difference in whiteness and color yield between conventional alkaline scouring and conventional bleaching. This finding supports the literature [2] regarding the different effects of alkaline scouring and bleaching on cotton pore structure.
The type of bleaching agent affected the darkness of the color. Although the whiteness of peroxide bleaching was higher, it provided a better color yield than sodium perborate. The pectinase–cellulase enzyme combination (P-NC) with a relatively much lower degree of whiteness gave a higher color yield than enzyme-bleaching-TAED combinations, except for the enzyme–hydrogen peroxide combination with 1 g/L TAED (P-PHP1). This finding can be attributed to the positive effect of cellulase.
The type of bleaching agent is effective on color yield. When enzyme-hydrogen peroxide-2 g/L TAED (P-HP2), enzyme-sodium percarbonate-TAED (P-SC), and enzyme-sodium perborate-TAED (P-SB) combinations were compared, it was concluded that the type of bleaching agent was effective and hydrogen peroxide resulted in higher color yield than sodium perborate but lower than sodium percarbonate. The concentration of TAED also created significant differences in color yield.
Figure 8 shows the relative weight loss and color yield with iron mordant.
Conventional scouring produced the highest weight loss, followed by conventional bleaching (99.1%). The combination of pectinase and cellulase resulted in higher weight loss than pectinase. Enzymatic scourings caused lower weight loss than conventional scouring and peroxide bleaching. Among the enzyme and bleaching combinations, P-HP2 (75.5%) had the highest and P-SC (73.1%) had the lowest weight loss values. P-SC and P-SB produced the lowest weight loss among the enzyme and peroxide bleaching combinations, with no significant difference between them. No definite conclusion could be reached, such as whether weight loss increases or color yield increases or decreases. For example, conventional scouring with the highest weight loss had a lower color yield than P-HP1 with a weight loss of 74.2%. The color yield of P-NC with a larger weight loss was 90%, whereas that of pectinase was 72%. This result does not agree with the study reports that vat dyes also showed an improvement in K/S, but it decreased with an increase in weight loss [40]. As extended hydrolysis reduces accessible regions, dyeing properties may be improved but then reduced with increasing weight loss. Removal of protruding fibers which decreases the scattering coefficient improves the color yield. If amorphous region decreases, the color yield is also expected to decrease [40].
No different dyeing affinity was obtained between enzymatic treated and untreated fabrics. Two types of regions accessible to dye molecules were suggested through enzyme action: readily digested areas and additionally developed regions. Dyeing affinity increased and then decreased with an increase in weight loss. This finding indicates that additionally developed accessible regions decrease with extended hydrolysis [45]. It can be considered that structural changes are also effective as weight loss. Differences in the results of this and previous studies suggest that the changes in the crystalline structure of the fiber caused by various processes should be considered as well as the removal of non-cellulosic impurities (weight loss).
P-HP1 (74.2%) and P-HP3 (74.6%) with almost identical weight losses produced significant color yield differences of 114 and 88%, respectively. A similar situation exists between enzyme-sodium perborate and enzyme-sodium percarbonate combinations. Sodium percarbonate and sodium perborate produced 85 and 71% color yields, respectively. The TAED concentration did not produce a distinct effect on weight loss.
Relative pectin removal and color yield with iron mordant are shown in Fig. 9. In general, absorbency increased as the pectin content decreased. Conventional bleaching resulted in the highest pectin content, whereas the pectinase–cellulase combination resulted in the lowest. When conventional scouring was compared with enzymatic scouring, conventional scouring contained more pectinic substances but provided the darkest color. However, the pectinase–cellulase combination with 86% pectin removal and pectinase with 87.5% pectin removal had 90 and 72% color yields, respectively. In enzyme-bleaching-TAED combinations, sodium perborate (P-SB) with the highest pectin content gave the lowest color yield. No definite relationship was established between pectin removal and color yield. Among all samples, the best pectin removal was achieved by alkaline pectinase-cellulase combination and the worst by conventional bleaching. No significant difference was found between sodium perborate and sodium percarbonate in terms of pectin removal. Considering conventional bleaching and enzyme-bleaching-TAED combinations, the use of enzyme slightly enhanced pectin removal.
3.6 Effect of Pretreatment on a *, b *, C * in Natural Dyeing with Iron Mordant
Figure 10 shows a*, b*, C* values with iron mordant. Pretreatment affected color coordinates. Enzyme-hydrogen peroxide bleaching in combination with 1 g/L TAED (P-HP1) created the greatest change in color coordinates and color yield. With reference to conventional scouring, enzymatic scouring showed no significant difference in color coordinates.
3.7 Effect of Pretreatment on Hue (h) in Natural Dyeing with Iron Mordant
When Fig. 5 (relative color yield) and Fig. 11 (hue) were compared, it was detected that the results showed a similar trend. P-HP1 provided the most different hue. This result agrees with the color yield evaluation. This was followed by P-HP3, which had the highest whiteness among the enzyme-bleaching combinations. P-HP3 provided the closest hue to conventional bleaching. Enzymatic scourings also had hue values close to those of conventional scouring. No significant differences were observed between enzymatic scourings, conventional scouring and conventional hydrogen peroxide bleaching. However, enzyme-hydrogen peroxide bleaching combinations resulted in higher hue differences. TAED concentration was also effective. Even if the whiteness of the fabrics is the same or very close, there may be a difference between their hue values.
3.8 Effect of Pretreatment on Color Yield (K/S) in Natural Dyeing with Alum Mordant and the Relationship Among Absorbency, Whiteness, Weight Loss, Pectin Removal, and Color Yield
Figure 12 shows the relative color yield with the alum mordant. The relative absorbency and color yield with alum mordant are shown in Fig. 13. The K/S values of conventionally pretreated fabrics can easily be reached by the enzymatically pretreated fabrics in reactive dyeing. Even higher color depth values can be obtained [36]. In this study, different results were obtained in bioscouring. The type of biopreparation seems to be important.
Conventional scouring followed by the pectinase–cellulase combination provided the best color yield. Although their absorbency properties were quite similar, there was a significant difference between the color yield of pectinase and the pectinase–cellulase combination. It exhibited the closest color yield to conventional scouring. The color yield of this combination was much higher than that of the enzyme-hydrogen peroxide-TAED combinations with higher absorbency. Therefore, no consistent relationship between absorbency and color yield could be established. P-HP3 and P-SC provided higher color yields than conventional bleaching. When enzyme-hydrogen peroxide bleaching combination with 2 g/L TAED (P-HP2), enzyme-sodium percarbonate (P-SC), and enzyme–sodium perborate (P-SB) were compared to evaluate the effect of the bleaching agent, it was observed that darkness increased with increasing absorbency. In addition, the type of bleaching agent was effective. The order from darkest to lightest color was enzyme–sodium percarbonate, enzyme-sodium perborate, and enzyme–hydrogen peroxide.
The type of mordant affected the darkening tendency and alum produced different results than the iron mordant. However, some results were similar. For example, the color yield of enzymatic scouring did not fully reach that of conventional scouring. The color yield of the pectinase-cellulase combination (90%) was much better than that of alkaline pectinase (62%) and was closer to that of alkaline scouring. This finding was similar to the result obtained using the iron mordant. Conventional scouring followed by the pectinase–cellulase combination gave the best color yields. There was a significant difference in darkness between pectinase itself and the pectinase-cellulase combination. This enzyme combination had better color yield than the enzyme–hydrogen peroxide-TAED combination. No definite relationship between absorbency and color yield has been established. In some samples, the one with the highest absorbency was dyed darker and vise versa.
Figure 14 demonstrates the relative (%) whiteness and color yield with the alum mordant. Although the whiteness of the enzyme combination was slightly better than that of alkaline pectinase, its color yield was significantly higher (90%). Conventional bleaching also had a higher whiteness and color yield than pectinase (72%). Conventional scouring resulted in the highest color yield, followed by the pectinase-cellulase combination (90%). Similar to the result obtained using iron mordant, the color yield of conventional bleaching, whose whiteness was significantly higher than that of conventional scouring, was lower. Alkaline pectinase (62%) and enzyme-hydrogen peroxide bleaching combined with 2 g/L TAED (P-HP2) gave the lowest color yields (63%). No consistent conclusion could be reached regarding the relationship between whiteness and color yield. Among the enzyme and peroxide combinations, the enzyme–hydrogen peroxide bleaching combination with 4 g/L TAED (P-HP3) ensured the best color yield. Although enzyme-sodium percarbonate (P-SC) and enzyme-sodium perborate (P-SB) had the same whiteness value, sodium percarbonate provided a better color yield. The type of bleaching agent can be considered as an effective factor. The enzyme–hydrogen peroxide–4 g/L TAED combination (P-HP3) with the second highest degree of whiteness exceeded the color yield of conventional bleaching. On the other hand, the pectinase–cellulase combination with a whiteness of 76.7% provided the closest color yield (90%) to conventional scouring. Similarly, alkaline pectinase scouring with the lowest whiteness of 75.7% generated the lowest color yield (62%), while enzyme-hydrogen peroxide-2 g/L TAED combination (P-HP2) with 97% whiteness also had the lowest color yield (63%).
When enzyme-hydrogen peroxide bleaching combination with 2 g/L TAED (P-HP2), enzyme-sodium percarbonate-TAED (P-SC), and enzyme-sodium perborate-TAED (P-SB) were compared, it was found that the type of bleaching agent was also effective in natural dyeing with alum like iron mordant. However, unlike the results obtained using iron mordant, in enzyme bleaching combinations, hydrogen peroxide gave lower color yields than both sodium perborate and sodium percarbonate. The color yield of the pectinase and neutral cellulase combination was higher than that of all trials except for conventional scouring.
The relative weight loss and color yield with alum mordant are shown in Fig. 15. Conventional scouring with the highest weight loss generated the highest color yield, whereas pectinase with the lowest weight loss (69.4%) had the worst color yield (62%). On the other hand, although their weight losses were quite close, the pectinase and pectinase–cellulase combination resulted in 62% and 90% color yields, respectively. This enzyme combination generated the best color yield. The enzyme mixture creates a synergistic effect. In addition, although the weight losses of conventional scouring and bleaching were almost the same, the color yield of conventional bleaching was much lower (72%). The type of process seems to have been more effective than weight loss. The weight loss values of enzyme-bleaching combinations exhibited no remarkable differences; however, their color yields were significantly different. In these trials, the enzyme–hydrogen peroxide bleaching combination with 2 g/L TAED (P-HP2) had the lowest color yield, whereas the color yields of enzyme-hydrogen peroxide with 4 g/L TAED (P-HP3) and enzyme-sodium percarbonate-TAED combinations (P-SC) exceeded those of conventional bleaching. Of all the enzymatic treatments, pectinase scouring with the lowest weight loss (69.4%) provided the lightest color.
Figure 16 presents the relative pectin removal and color yield with the alum mordant. Enzymatic scouring provided better pectin removal than conventional scouring and bleaching. Of all the samples, pectinase resulted in the lowest color yield. The pectinase-cellulase combination had the lowest pectin content. Although their pectin removal values were similar, a significant difference occurred between their color yields. Pectinase with 87.8% pectin removal achieved 62% darkness, whereas pectinase–cellulase combination with 86% pectin removal resulted in 90% darkness. Conventional scouring with 89.8% pectin removal had the highest color yield. No general conclusion could be reached regarding the relationship between pectin removal and color yield. A sample with a high pectin content can also be dyed lighter or darker. In enzyme–peroxide–TAED combinations, it was observed that color yield increased as pectin removal increased. Of all the samples, pectinase resulted in the lowest color yield. Although enzyme-sodium percarbonate-TAED (P-SC) and enzyme-sodium perborate-TAED (PSB) combinations had very near pectin removal, sodium percarbonate achieved a higher color yield.
3.9 Effect of Pretreatment on a *, b *, C * in Natural Dyeing with Alum Mordant
Figure 17 indicates a*, b*, and C* values with alum mordant. No significant differences were observed between the color coordinates of enzyme-bleaching-TAED combinations and conventional bleaching. Unlike dyeing with iron, the a*b*C* values of conventional scouring and enzymatic scourings were obviously different, and alkaline pectinase scouring created a larger difference in color coordinates than conventional scouring and the pectinase–cellulase combination. There was also a difference between the pectinase and pectinase-cellulase combination.
3.10 Effect of Pretreatment on Hue (h) in Natural Dyeing with Alum Mordant
The relative (%) hue values with alum are shown in Fig. 18. The pectinase–cellulase combination provided a closer hue to conventional scouring than pectinase. Enzyme-hydrogen peroxide-4 g/L TAED (P-HP3) and enzyme-sodium percarbonate-TAED (P-SC) had the closest hue values to conventional scouring. Enzyme-hydrogen peroxide-1 g/L TAED (P-HP1) and enzyme-sodium perborate-TAED (P-SB) had the most similar hues to conventional bleaching. Among the enzyme-bleaching combinations, the enzyme-hydrogen peroxide bleaching combination with 4 g/L TAED (P-HP3) had the highest whiteness value, whereas sodium percarbonate (P-SC) and sodium perborate (P-SB) had the lowest whiteness. There was also a significant difference between the whiteness of conventional scouring (85.8%) and conventional bleaching (100%). Therefore, the hue was also significantly different. However, the whitenesses of enzymatic scourings (75.7 and 76.7%) were significantly lower than those of conventional bleaching, and their hue values were significantly different. Although the whiteness values of enzymatic scourings were very close, the hue values were significantly different. Among the enzyme and bleaching combinations, the hue values of the P-HP1 and P-SB samples were closest to those of conventional bleaching. Bleaching agent and TAED were significantly effective.
3.11 Wash Fastness
The type of pretreatment did not affect washing fastness. There was no difference between the washing fastness (4–5 for staining and color change) of the trials. This finding is consistent with other studies reporting that excellent wash fastness values were achieved regardless of the type of pretreatment [3, 12, 36, 46].
3.12 Common Findings of Natural Dyeing with Iron and Alum Mordants
Type of mordant had a great impact on darkness and color coordinates. The best color yields were obtained in conventional scouring except for the enzyme-hydrogen peroxide bleaching combination with 1 g/L TAED (P-HP1). The pectinase-cellulase combination provided significantly better color yield than alkaline pectinase scouring. The color yield of conventional scouring could not be exactly reached by enzymatic scourings. Although enzymatic scouring did not achieve color yields equal to or higher than conventional scouring, a very close result was obtained with the pectinase-cellulase combination. In the case of enzymatic scouring-bleaching-TAED combinations, different and more variable results occurred. The color yield of conventional bleaching was higher or lower than that of some enzyme-bleaching-TAED combinations. Enzyme-sodium perborate (P-SB) resulted in a lower color yield than enzyme-sodium percarbonate (P-SC). When P-HP2, P-SC, and P-SB were compared, it was found that the type of bleaching agent was effective.
4 Conclusions
Conventional scouring had slightly higher absorbency than enzymatic scourings. Alkaline pectinase-cellulase combination provided absorbency much closer to conventional scouring due to the synergistic effect of the enzymes. Despite very close absorbency values, a significant color yield difference was found between pectinase and the pectinase-cellulase combination. The color yield of this combination was much higher than that of the enzyme-hydrogen peroxide-TAED combinations with higher absorbency.
Although only the water absorbency of the enzyme-hydrogen peroxide-2 g/L TAED combination (P-HP2) was slightly lower, the other enzyme-bleaching-TAED combinations ensured the best water absorbency values compared with conventional bleaching. The whiteness of biopreparation processes was slightly lower, but it did not create a negative effect on color properties. Enzyme-hydrogen peroxide-4 g/L TAED combination (P-HP3) provided the closest whiteness to conventional bleaching. Increase in TAED concentration improved whiteness very slightly. Conventional and pectinase scouring had the highest and lowest weight losses, respectively. In all cases, biopreparation resulted in lower weight loss than conventional processes. Since TAED concentration and type of bleaching agent did not significantly affect weight loss, no remarkable differences were observed among enzyme-bleaching-TAED combinations. All biopreparation processes had advantages over conventional scouring and bleaching in terms of pectin removal and weight loss. Enzymatic treatments can improve the color yield. Dyeing results equivalent to conventional scouring with enzymatic bioscouring could not be obtained, but the pectinase–cellulase combination produced a very close color yield to conventional scouring. The difference between them was imperceptible. In the case of enzyme-bleaching-TAED combinations, the use of enzyme increased the color yield compared with conventional bleaching. The color yield of enzymatic scouring and enzyme-bleaching-TAED combination may not reach conventional scouring and bleaching depending on factors such as type of enzyme, dye, and mordant. The color yield of pectinase scouring was lower than that of conventional scouring. However, the pectinase-cellulase combination exceeded that of conventional bleaching and enzyme-bleaching-TAED combinations, except for P-HP1 in natural dyeing with iron mordant. Moreover, the color yields of the other enzyme-bleaching-TEAD combinations were equivalent to or higher than those of conventional bleaching, although only the sodium perborate combination was slightly lower. In the case of dyeing with alum, the pectinase-cellulase enzyme combination achieved higher color yields than conventional bleaching and all enzyme-bleaching-TAED combinations. In addition, the color yields of the other enzyme-bleaching-TAED combinations were equivalent to or higher than those of conventional bleaching, although only P-HP2 was slightly lower.
Absorbency, whiteness, pectin removal, and weight loss alone are not distinct determinants of color yield and color coordinates. Without exception, iron mordant resulted in much darker colors than alum in all trials. Considering this study and other references, biopreparation and dyeing results vary depending on factors such as the type of process, dye, fiber and enzyme, source of enzyme and process conditions. Therefore, whiteness, water absorbency, weight loss and removal of non-cellulosic impurities influenced natural dyeing results and their relationships also changed. The findings of some researchers also support these observations. Excellent wash fastness values were achieved regardless of the type of pretreatment. Energy, water, time, and cost-saving one-step low-temperature biopreparation can be a viable alternative to conventional scouring and bleaching processes. However, it is of great importance to determine the type of biopretreatment and recipe depending on the end-use properties of the textile (e.g. whether it will be white or dyed/printed, its color and lightness/darkness) at the laboratory and industrial scale applications.
5 Future Prospects
Today, natural dyeings have gone far beyond the traditional applications as a result of scientific and technological developments and interdisciplinary studies between different disciplines such as chemistry, physics, biology, biotechnology, electrical-electronics [47]. Considering that the textile industry is one of the most environmentally damaging sectors, the importance of researching and developing production methods that consume the least energy, water, dyes, chemicals and auxiliaries is evident. In addition, since energy consumption has a significant share in environmental impact and production cost, dyeing, printing and finishing processes should be carried out at the lowest possible temperatures.
In natural dyeing, it has been shown that time and energy saving, elimination of some chemicals, and higher extraction and dyeing efficiency are achieved by using environmentally friendly methods such as ultrasound, plasma, ozone, enzyme, microwave, gamma rays, UV rays [48,49,50,51,52,53,54,55]. However, the applications of these technologies in textile mills are limited. Although laboratory studies on the mentioned subjects are very important, it would also be useful to carry out R&D studies for systems in which these information and technologies can be realized at the industrial scale productions. In addition, detailed comparisons can be made by analyzing the energy, water, chemical consumption and cost of the new processes to be proposed as an alternative to conventional ones from a scientific and holistic point of view. New methods and technologies can enable natural dyed textiles to be produced in a more efficient, high quality and sustainable manner. These technologies can include innovative solutions such as biotechnology, the use of microorganisms, enzyme technology and nanotechnology. Furthermore, industrial-scale applications of natural dyes not only offer environmental advantages but could also gain popularity among consumers. In recent years, the demand for environmentally friendly products has increased and this trend encouraged the industrial use of natural dyes. Sustainability and ecological approaches are expected to become widespread. This is a positive development for both the environment and consumers.
Data Availability
The author(s) declare that the data supporting the findings of this study are available within the paper.
References
Ö. Erdem İşmal, AATCC Rev. 8, 37 (2008)
Y.L. Hsieh, J. Thompson, A. Miller, Text. Res. J. 66, 456 (1996)
A. Losonczi, E. Csiszar, G. Szakacz, Text. Res. J. 74, 501 (2004)
M. Kumar, S.R. Shukla, A. Arputharaj, S. Saxena, S. Patil, P.G. Patil, E. Varghese, R. Amarowicz, Fibers Polym. 22, 2803 (2021)
L. Cui, P. Wang, Q. Wang, X. Fan, Fibers Polym. 10, 476 (2009)
M.M. Hassan, K. Saifullah, Fibers Polym. 20, 578 (2019)
C. Guo, T. Li, C. Wang, Y. Wang, Y. Zhang, Fibers Polym. 18, 1882 (2017)
N. Špička, Ž Zupin, J. Kovač, P.E.F. Tavčer, Fibers Polym. 16, 1723 (2015)
N.A. Ibrahim, B.M. Eid, M.S. Abdel Aziz, S.M. Hamdy, S.E. AbdAllah, Fibers Polym. 20, 787 (2019)
A. Singh, A. Kaur, A.K. Patra, R. Mahajan, 3 Biotech. 8, 184 (2018)
F. Darinka, G. Darko, S. Zoran, Fibres Text. East. Eur. 67, 101 (2008)
Ö. Erdem İşmal, A.T. Özgüney, A. Arabacı, AATCC Rev. 7, 34 (2007)
F. Sia, K. Yana, X. Zhang, Carbohydr. Polym. 103, 581 (2014)
J.Y. Cai, D.J. Evans, S.M. Smith, AATCC Rev. 1, 31 (2001)
A. Hou, X. Zhang, Y. Zhou, Carbohydr. Polym. 82, 618 (2010)
S.H. Lim, D. Hinks, P. Hauser, Text. Res. J. 74, 970 (2004)
S. Lim, J.J. Lee, D. Hinks, P. Hauser, Color. Technol. 121, 89 (2005)
J. Shao, Y. Huang, Z. Wang, J. Liu, Color. Technol. 126, 103 (2010)
C. Xu, R. Shamey, D. Hinks, Cellulose 17, 339 (2010)
C. Xu, X. Long, J. Du, S. Fu, Carbohydr. Polym. 92, 249 (2013)
D. Phillips, J. Scotney, AATCC Rev. 2, 50 (2002)
E.U. Çelik, M. Türkün, A.G.D. Yapar, Int. Endod. J. 41, 571 (2010)
Y. Liu, J. Tao, J. Sun, W. Chen, Carbohydr. Polym. 112, 416 (2014)
A. Hebeish, M. Hashem, N. Shaker, M. Ramadan, B. El-Sadek, M. Abdel Hady, Carbohydr. Polym. 78, 961 (2009)
S. Shakouie, A.S. Milani, M. Eskandarnejad, S. Rahimi, M. Froughreyhani, S. Galeda, E. Ranjbar, J. Dent. Res. 10, 43 (2016)
X. Long, C. Xu, J. Du, S. Fu, Carbohydr. Polym. 95, 107 (2013)
S.J. Scarborough, A.J. Mathews, AATCC Rev. 32, 33 (2000)
Y.M. Indi, A. Wasif, Indian J. Fibre Text. Res. 43(1), 120 (2018)
Q. Li, R. Lu, Y. Liang, K. Gao, H. Jiang, Materials 15, 1 (2022)
Y. Dong, L. Bian, C. Zhang, B. Li, Color. Technol. 136, 389 (2020)
E. Vujasinovi, A. Tarbuk, T. Pušic, T. Dekanic, Processes 11(1), 103 (2023)
Ö. Erdem İşmal, L. Yıldırım, Indian J. Fibre Text. Res. 37, 358 (2012)
Ö. Erdem İşmal, E. Özdoğan, L. Yıldırım, Color. Technol. 129, 431 (2013)
Ö. Erdem İşmal, L. Yıldırım, E. Özdoğan, J. Cleaner Prod. 70, 61 (2014)
Ö. Erdem İşmal, L. Yıldırım, E. Özdoğan, J. Text. Inst. 106, 343 (2015)
Ö. Erdem İşmal, A.T. Özgüney, A. Arabacı, AATCC Rev. 7, 39 (2007)
C. Li, C.M. Ladisch, M.R. Ladisch, Text. Res. J. 71, 407 (2001)
G. Buschle-Diller, M.K. Traore, Text. Res. J. 68, 185 (1998)
H. Koo, M. Ueda, T. Wakida, Text. Res. J. 61, 70 (1993)
M.G. Uddin, Trends. Green Chem. 2, 1 (2016)
D. Saravanan, N.S. Vasanthi, T. Ramachandran, Carbohydr. Polym. 76, 1 (2009)
M.K. Traore, G. Buschle-Diller, Text. Chem. Color. Am. Dyest Rep. 1(4), 51 (1999)
A. Hebeish, M.M. Kamel, H.M. Helmy, N.S. El Hawary, Life Sci. J. 10, 3281 (2013)
A. Hebeish, M.M. Kamel, H.M. Helmy, N.S. El Hawary, Egypt. J. Chem. 56, 367 (2013)
R. Mori, T. Haga, T. Takagishi, J. Appl. Polym. Sci. 59, 1263 (1996)
A. Madhu, J. Chakraborty, Fibers Polym. 23, 993 (2022)
Ö. Erdem İşmal, Yedi J Art Des Sci 22, 41 (2019)
E. Kalaycı, A. Yavaş, O. Avinç, in Natural Dyes and Sustainability. ed. by S.S. Muthu (Springer, New York, 2024), p.329
A. Yavaş, O. Avinç, G. Gedik, Fibres Text. East. Eur. 25, 111 (2017)
H. Benli, M.İ Bahtiyari, Cellulose 22, 867 (2015)
H. Benli, M.İ Bahtiyari, Ozone Sci. Eng. 40, 141 (2018)
A. Erdem, M.İ Bahtiyari, J. Cleaner Prod. 188, 670 (2018)
N. Amin, S. Adeel, F. Rehman, M.N. Anjum, Environ. Sci. Pollut. 30, 112825 (2023)
M. Azeem, S. Adeel, N. Habib, F. Rehman, A.F. Zahoor, M. Saeed, N. Amin, S. Liaqat, M. Hussaan, Tekst ve Konfeksiyon 29, 181 (2019)
S. Shahidi, B. Moazzenchi, Fibers Polym. 20, 1658 (2019)
Acknowledgements
Novozymes is gratefully acknowledged for supplying enzymes.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author(s) declared no potential conficts of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erdem İşmal, Ö. Insight into Low-Temperature One-Step Biopreparation and Natural Dyeing of Cotton as an Environmentally Benign Route. Fibers Polym 25, 2185–2202 (2024). https://doi.org/10.1007/s12221-024-00561-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-024-00561-0