Abstract
For conjugating sulfa drug moieties with Schiff’s bases scaffold in the same build through an azo linker to take advantage of the bioactive feature of both motifs, we designed and synthesized a series of bioactive disperse dyes. The target disperse dyes, methyl 2-(E-2-hydroxy-5-((E)-(4-sulfa-derivative) diazenyl)benzylidene) hydrazine-1-carbodithioates 4a–e have been synthesized via the acidic reaction of azo dyes 3a–e with methyl hydrazine carbodithioate. Structures of the synthesized dyes were clarified based on their spectral and elemental analyses. The effectiveness of the dyes was initially tested as an antibacterial toward Staphylococcus aureus ATCC 6538-P and Escherichia coli ATCC 25933. Dyes that were proven to be effective against bacteria have been used as disperse dyes to print polyester fabrics. The color properties of the dyes and their fastness properties counting washing, perspiration, light, rubbing, and sublimation fastness were also examined. The printed polyester fabrics were evaluated for their antibacterial activity via colony-forming unit (CFU) technique. Fabric samples treated with 4c, 4d, and 4b had promising anti-Gram-positive activities against S. aureus. Whereas 4c-, 4d-, and 4b-treated fabrics exhibited moderate anti-Gram-negative activities against the test bacterium E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Textile printing is the process of applying colors to fabric with specific patterns or designs in contrast to the dying process [1, 2]. As a result, there are some factors that affect the choice of dyes used for printing, such as choosing the type of dyes as well as the amount of dye used [1, 2]. Meaning that they are affected by the thickener used, the composition of the printing paste (thick and sticky), the composition of the fibers as well as the design of the fabric to be printed [1, 2].
Polyester fibers are considered cheap and readily obtainable raw materials with suitable properties, e.g., high strength, lightweight with excellent dye ability [3]. Azo disperse heterocyclic dyes are commonly applied for dyeing and printing polyester [4,5,6]. They have good brilliance colors, perfect tinctorial thickness with excellent fastness properties [4,5,6].
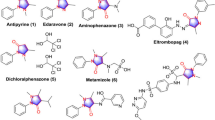
Azo dyes are one of the most widely used chromophores in dye chemistry [7]. Azo dyes have brilliant colors in the yellow to blue–green region adjustable by varying between azo components with electron-withdrawing groups and coupling components with electron-donating groups [7, 8]. Attention to the preparation of azo dye based on sulfa drugs started since long time ago due to their high degree of brightness compared to azo dyes derived from aniline [9]. Besides coloring properties of azo-day based on sulfa drugs, their biological activities are known, for example, antimicrobial [10,11,12,13,14], antioxidant [15], anti-inflammatory [16], anti-mycobacterial [17], and anticancer [17,18,19] (Fig. 1). Prontosil, a red azo dye, is the first sulfonamide-based antibacterial drug that was made available to the public in 1932 [20]. In addition, triple sulfa® drug, combination of sulfadiazine, sulfamerazine, and sulfamethazine, was used for the treatment of bacterial vaginosis [21].
Recently, many researchers have focused on the creation of disperse azo dyes, examined their antibacterial activity, and reported their application in polyester printing [4, 22,23,24]. For instance, Gaffer et al. have synthesized and evaluated the antibacterial activity of some new thiazole azo disperse dyes containing sulfaguanidine and sulfadiazine and reported their application in polyester printing [23]. In the same manner, in 2022, Ragab et al. generated dispersed antimicrobial dyes by coupling a 2-spirocyclic 2-thiopyrimidine scaffold with a sulfa drug moiety through an azo linker to take advantage of the bioactivity of both forms [15].
The present work aimed to design and synthesize simple disperse dyes to be used as polyester printing and evaluate their antibacterial activity.
2 Experimental
2.1 Chemicals and Reagents
All reagents and solvents were of commercial grade. Sulfa drugs were obtained from Sigma-Aldrich Chemie GmeH, Taufkirchen, Germany. Sodium dihydrogen phosphate (NaH2PO4, 98.0%) and sodium lignosulphonate 99.5% were obtained from LOBA Chemie, Pvt Ltd, India. Acrylate copolymers thickener and LyPrint (sodium salt of nitrobenzene sulfonic acid) were delivered from BASF Company. Polyester (150 g/m2) was obtained from the Egyptian and Developing Company, Cairo, Egypt. A synthetic thickener for azo disperse silk screen printing (Daico-Thick-1600) was supplied by Daico Company.
2.2 Instrumentation of Spectral Analyses and Melting Points
Melting points were determined on a digital melting point apparatus (Electro thermal 9100, Electro thermal Engineering Ltd, serial No. 8694, Rochford, United Kingdom) and are uncorrected. The reaction progress was monitored by thin layer chromatography (TLC) using silica gel plates (POLYGRAM SILG/UV254, 0.20 mm), which were visualized under UV light 254 and 365 nm. Elemental analyses were carried out on a Perkin-Elmer 2400 analyzer (USA), and were found within ± 0.4% of the theoretical values. 1H- and 13C-NMR spectra were measured with a Jeol spectrometer Japan at 500 and 125 MHz, respectively, using TMS as the internal standard. Hydrogen coupling patterns are described as (s) singlet, (d) doublet, (t) triplet, (q) quartet, and (m) multiplet. Chemical shifts were defined as parts per million (ppm) relative to the solvent peak.
2.3 Synthesis
2.3.1 General Procedure for the Preparation of Azo Dye Ligands 3a–e
To a suspended solution of sulfa drugs (1a–e, 1.85 mmol) in HCl (10 ml, 6N), ice-cold solution of sodium nitrite (NaNO2, 3.7 mmol, 2.56 g, 2 ml) was added slowly over 30 min at 0 °C. The resulting solution of diazonium salt was added slowly to the cold solution of sodium salt of salicylaldehyde, [(3.7 mmol, 1.6 g) NaOH, (1.85 mmol, 2.56 g) salicylaldehyde) in ethanol (5 ml), 1 ml (H2O)], with stirring at 0–5 °C until the product was precipitated. The solid precipitate was filtered, washed several times with water, air dried, and recrystallized from ethanol:DMF (5:1) to give the pure 3a–e.
The azo dye ligands 3a–d were prepared by the coupling of sulfanilamide, sulfaguanidine sulfamethoxazole, and sulfadiazine diazonium salts with salicylaldehyde according to the previously reported methods 3a [25], 3b [26, 27], 3c [14], and 3d [28] with yield 55–66%.
(E)-N-(4,6-Dimethylpyrimidin-2-yl)-4-((3-formyl-4-hydroxyphenyl)diazenyl) benzenesulfonamide (3e). Brown powder; MP. 204–6 °C; yield: 5.85 g, 77%; 1H-NMR (500 MHz, DMSO-d6) δ 11.62 (s, 1H, exchangeable with D2O), 10.33 (m, 1H, CHO), 8.17 (s, 1H, exchangeable with D2O), 8.12 (d, J = 8.3 Hz, 1H), 8.10 – 8.07 (m, 2H), 8.07 (d, J = 2.4 Hz, 1H), 7.93 (d, J = 8.4 Hz, 2H), 7.19 (d, J = 8.4 Hz, 1H), 6.71 (s, 1H), 2.22 (s, 6H, 2-CH3); 13C-NMR (125 MHz, DMSO-d6) δ 191.0, 164.4, 156.5, 154.2, 145.4, 130.4, 129.9, 125, 123.2, 122.6, 119.1, 113.4, 23.1 (2-CH3); Anal. Calcd for C19H17N5O4S (411.44): C, 55.47; H, 4.16; N, 17.02; S, 7.79; found: C, 55.22; H, 4.32; N, 16.98; S, 7.65.
2.3.2 General Procedure for the Synthesis of Schiff’s Bases 4a–e
A mixture of sulfonamide derivatives (3, 5 mmol) and methyl hydrazine carbodithioate (5 mmol, 0.6 g) in absolute ethanol (5 ml) containing two drops of glacial acetic acid was stirred at room temperature for 30 min. The obtained solid was filtered off, air dried, and recrystallized from ethanol: DMF to obtain pure products 4a–e.
Methyl 2-((E)-2-hydroxy-5-((E)-(4-sulfamoylphenyl)diazenyl) benzylidene) hydrazine-1-carbodithioate (4a). Yellow crystals; MP. 169–71 °C; yield: 1.4 g, 66%; 1H-NMR (500 MHz, DMSO-d6) δ 13.3 (s, 1H, exchangeable with D2O), 11.1 (s, 2H, exchangeable with D2O), 8.59–7.9 (m, 8H, Ar–H), 4.21 (s, 1H, exchangeable with D2O), 2.64 (s, S-CH3); 13C-NMR (125 MHz, DMSO-d6) δ 198.4, 161.2, 154.1, 145.9, 145.7, 143.5, 127.2, 123.5, 117.9, 17.3 (S-CH3); Anal. Calcd for C15H15N5O3S3 (409.50) C, 44.00; H, 3.69; N, 17.10; S, 23.49; found: C, 43.89; H, 3.55; N, 17.22; S, 23.32.
Methyl 2-((E)-5-((E)-(4-(N-carbamimidoylsulfamoyl)phenyl) diazenyl)-2-hydroxybenzylidene)hydrazine-1-carbodithioate (4b). Yellow crystals; MP. 243–5 °C; yield: 1.6 g, 70%; 1H-NMR (500 MHz, DMSO-d6) δ 13.40 (s, 1H, exchangeable with D2O), 11.83 (s, 1H, exchangeable with D2O), 11.21 (d, J = 15.0 Hz, 1H, exchangeable with D2O), 8.55 (d, J = 15.5 Hz, 1H), 8.30 (d, J = 15.5 Hz, 1H), 8.01 – 7.90 (m, 5H), 7.10 – 7.03 (m, 2H), 6.80 (s, 2H), 2.63 (s, 3H, S-CH3); 13C-NMR (125 MHz, DMSO-d6) δ 198.4, 161.1, 158.7, 153.6, 146.5, 145.7, 143.4, 127.5, 126.2, 123.4, 123.0, 120.8, 117.9, 17.3 (S-CH3); Anal. Calcd for C16H17N7O3S3 (451.54) C, 42.56; H, 3.80; N, 21.71; S, 21.30; found: C, 42.44; H, 3.67; N, 21.55; S, 21.12.
Methyl 2-((E)-2-hydroxy-5-((E)-(4-(N-(5-methylisoxazol-4-yl)sulfamoyl)phenyl) diazenyl)benzylidene)hydrazine-1-carbodithioate (4c). Yellow crystals; MP. 237–9 °C; yield: 1.8 g, 75%; 1H-NMR (500 MHz, DMSO-d6) δ 13.40 (s, 1H, exchangeable with D2O), 11.62 (s, 1H, exchangeable with D2O), 11.27 (s, 1H, exchangeable with D2O), 10.33 (s, 1H, CH=N), 8.57 (d, J = 15.5 Hz, 1H), 8.29–7.97 (m, 5H), 7.20–7.10 (dd, 1H), 6.14 (s, 1H), 2.46 (s, 3H, S-CH3), 2.27 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6) δ 198.5, 190.9, 171.1, 164.6, 161.1, 157.9, 154.8, 145.8, 143.3, 141.2, 130.4, 128.8, 126.4, 125.1, 123.3, 117.8, 96.1, 17.3 (S-CH3), 12.6 (CH3); Anal. Calcd for C19H18N6O4S3 (490.57) C, 46.52; H, 3.70; N, 17.13; S, 19.61; found: C, 46.44; H, 3.56; N, 17.01; S, 19.76.
Methyl 2-((E)-2-hydroxy-5-((E)-(4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl) diazenyl)benzylidene)hydrazine-1-carbodithioate (4d). Yellow crystals; MP. 248–50 °C; yield: 1.7 g, 70%; 1H-NMR (500 MHz, DMSO-d6) δ 13.41 (s, 1H, exchangeable with D2O), 12.01 (s, 1H, exchangeable with D2O), 11.25 (s, 1H, exchangeable with D2O), 8.58 (s, 1H), 8.48 (d, J = 4.7 Hz, 2H), 8.29 (d, J = 2.2 Hz, 1H), 8.12 (d, J = 8.4 Hz, 1H), 7.95 (d, J = 8.5 Hz, 2H), 7.89 (dd, J = 8.8, 2.3 Hz, 2H), 7.11–7.03 (dd, J = 36.4, 6.7 Hz, 2H), 2.62 (s, S-CH3); 13C-NMR (125 MHz, DMSO-d6) δ 198.5, 161.4, 158.9, 157.3, 154.6, 145.8, 143.3, 142.2, 129.5, 126.4, 123.5, 123.1, 120.9, 117.9, 116.3, 17.3 (S-CH3); Anal. Calcd for C19H17N7O3S3 (487.57) C, 46.81; H, 3.51; N, 20.11; S, 19.73; found: C, 46.76; H, 3.41; N, 20.32; S, 19.68.
Methyl 2-((E)-5-((E)-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl) diazenyl)-2-hydroxybenzylidene)hydrazine-1-carbodithioate (4e). Yellow crystals; MP. 250–2 °C; yield: 1.8 g, 71%; 1H-NMR (500 MHz, DMSO-d6) δ 13.3 (s, 1H, exchangeable with D2O), 11.27 (s, 1H, exchangeable with D2O), 9.80 (s, 1H, CH=N), 8.12 (d, J = 8.3 Hz, 1H), 8.10–8.07 (m, 3H), 8.07 (d, J = 2.4 Hz, 2H), 7.93 (d, J = 8.4 Hz, 2H), 6.71 (s, 1H), 2.46 (s, 3H, S-CH3), 2.22 (s, 6H, 2-CH3); 13C-NMR (125 MHz, DMSO-d6) δ 198.0, 172.6, 164.4, 156.5, 154.2, 145.4, 130.4, 129.9, 125, 123.2, 122.6, 119.1, 110.4, 23.1 (2-CH3), 17.3 (S-CH3); Anal. Calcd for C21H21N7O3S3 (515.63) C, 48.92; H, 4.11; N, 19.02; S, 18.65; found: C, 48.85; H, 4.01; N, 18.98; S, 18.47.
2.3.3 Preparation of Printing Paste
The proportions used to create the constituent parts of the printing paste are tabulated in Table 1. Silkscreen printing samples of polyester fabrics with the aforementioned printing paste were done using the standard screen printing methodology [29]. The prints were allowed to dry at room temperature before undergoing for 4 min at 180 °C thermo-fixation. The prints were subjected to different washing processes: twice in cold water, twice in hot water, twice in cold water again, 60 °C for 30 min, 2 g/l nonionic detergent, and air dried.
2.4 Color Measurements
The colorimetric strength data were measured upon soaping of the printed polyester fabric samples by light reflectance technique using a Hunter Lab Ultrascan PRO spectrophotometer. The soaping of the printed samples assumes that the K/S values are proportionate to the dye concentration on the fiber under the printing conditions used, at least at the concentration of dyes used (2.0 g in the paste). The color strength was assessed using the Kubelka–Munk as K/S (Eq. (1)) [30].
where R = decimal fraction of the dyed fabricreflection, K = absorption coefficient, and S = scattering coefficient.
2.5 Fastness Testing
Using standard ISO methodologies, such as rubbing, washing, perspiration, light, and sublimation, the fastness properties of the printed samples were evaluated [15, 31, 32]. The wash fastness test in accordance with ISO 105-C06 B2S [33] was done using 25 steel balls, 4.0 g/l ECE detergent, and 1.0 g/l sodium perborate at 50 °C for 30 min with a 50:1 liquor ratio. The rubbing fastness test and the perspiration fastness test, respectively, were carried out in accordance with ISO 105-X12 [34] and ISO 105-E04 [35]. Light fastness was assessed in accordance with ISO 105-B02 [36] using a xenon arc lamp, and sublimation fastness was tested in accordance with ISO 105P01 [37] using a fixometer at 180 °C and 210 °C.
2.6 Antibacterial Activity Screening of Synthesized Azo Compounds
The antibacterial activity of prepared azo compounds was established by in vitro against two pathogenic bacterial test microbe namely Staphylococcus aureus ATCC 6538 (G+ve) and Escherichia coli ATCC 25922 (G−ve). Each sample (5 mg) was dissolved in 2 ml of DMSO, and then 100 μl (containing 250 μg) was used to evaluate their antibacterial activity by cup plate diffusion method. Nutrient agar plates (10 cm diameter) were uniformly inoculated with 50 μl of 105–106 cells/ml from each bacterial stock cultures. A 1 cm diameter hole was made in media by gel cutter (Cork borer) in a sterile condition. One drop of melted agar was poured into the bottom of the hole and allowed to solidify to make a base layer. A total of 100 μl of dissolved samples were poured into the holes. The plates were kept at a low temperature (4 °C) for 2–4 h, to allow maximum diffusion. The plates were incubated at 37 °C for 24 h in an upright position to prevent the scattering of liquid samples and allow maximum growth of the organisms. The antibacterial activity of the tested samples was inspected by measuring the diameter of the inhibition zones expressed in millimeters (mm). The experiment was carried out twice and the mean of the reading was recorded [38].
2.7 Assessment of Antibacterial Activity of the Dyed Fabrics Using Colony-Forming Unit (CFU)
Fabric samples treated with synthesized compounds 4a, 3e, 4d, 4b, and 4c were subjected to evaluate their antibacterial activities by colony-forming unit (CFU) procedure [39]. The used test bacterial strains were Staphylococcus aureus ATCC 6538-P (G+ve bacterium) and Escherichia coli ATCC 25933 (G−ve bacterium). Bacterial stocks (100 μl of stock of CFU value of about108) were inoculated into a 20 ml freshly prepared liquid nutrient broth containing 5 g/l peptone; 3 g/l beef extract at pH 6.8 in 100 ml-volume of Erlenmeyer flasks and incubated for 24 h. Fabrics each of about 250 mg were added to the bacterial inoculated medium in 100 ml conical flasks. Each flask had 10 ml culture medium and inoculated by 20 μl of bacterial inoculums leaving the control (inoculated flasks without samples). After 24 h incubation at 37 °C, a serial dilution from each sample-containing culture and the controls has been done (10–1–10–4). The microbial inhibition was determined by the colony-forming units (CFU) through inoculating Petri dishes containing solidified nutrient agar medium with 50 μl from each dilution and calculating the reduction growth (R%) for treated samples in relation to control (untreated) according to the equation;
where A is CFU/ml for treated sample after 16 h incubation and B is CFU/ml for untreated sample after the same period of incubation time. The optical density of the incubated liquid culture medium was recorded at 600 nm. The greater the growth, the higher the turbidity, and the optical density figure was, therefore, directly proportional to the number of bacteria in the medium.
3 Results and discussion
Aiming to synthesize Schiff’s bases inspired of sulfa drug, azo dye ligands 3a–d were prepared by the coupling of sulfanilamide, sulfaguanidine sulfamethoxazole, and sulfadiazine diazonium salts with salicylaldehyde according to the previously reported methods 3a [25], 3b [26, 27], 3c [14], and 3d [28] with yield 55–66%. In the same manner, the azo-dye (E)-N-(4,6-dimethylpyrimidin-2-yl)-4-((3-formyl-4-hydroxyphenyl) diazenyl)benzenesulfonamide (3e) was prepared by the coupling of sulfadimidine diazonium salt with salicylaldehyde as illustrated in Scheme 1.
Acid catalyzed reaction of azo-day ligands 3a–e with the prepared methyl hydrazine carbodithioate [40] in absolute ethanol afforded the corresponding Schiff’s bases 2-((E)-2-hydroxy-5-((E)-(4-sulfa-derivative)diazenyl)benzylidene) hydrazine-1-carbodithioates 4a–e (Scheme 1). 1H-NMR spectra of 4a–e revealed three singlet signals exchangeable with D2O for OH, NH–SO2, and NH of methyl hydrazine carbodithioate, in addition to singlet signals at ~ 2.46 for S-CH3. Their 13C-NMR confirmed their structures via the presence of a peak at 17.3 ppm for all (4a–e) besides the rest of carbon of molecules.
All synthesized azo compounds were preliminary tested in vitro antibacterial activity to select the desired disperse dyes with antibacterial activity to produce pastes for printing polyester fabrics. The antibacterial activity was done against Staphylococcus aureus ATCC 6538 (Gram-positive bacterium), Escherichia coli ATCC 25922 (Gram-negative bacterium), using the cup plate diffusion method at a single dose of 250 μg/100 μl. From Table 2 and Fig. 2, it has been found that, compounds 3e, 4a, 4b, 4c, and 4d exhibited remarkably high antibacterial activity against the G+ve test microbe (S. aureus) with clear zone values increase in order of (4a) 23 < (4b) 24 < (4d) 26 < (3e) 28 < (4c) 34 mm, respectively. The rest of these compounds showed considerably moderate activities when tested against the same test microbe with clear zone values of 19, 14, 21, 20, and 17 mm, for compounds 3a, 3b, 3c, 3d, and 4e, respectively. When azo compounds were tested against the G−ve bacterium (E. coli), it is obvious that approximately the same trend has been found. Compounds 3a, 3e, 4a, 4b, 4c, 4d, and 4e exhibited appreciable high activities with clear zone values of 20, 23, 24, 19, 22, 21, and 20 mm, respectively. Compounds 3b, 3c, and 3d had moderate activities with clear zone values of 18, 17, and 18 mm, respectively.
According to the presented antibacterial activity results, compounds 3e, 4a, 4b, 4c, and 4d were selected for further study by applying them to textile fabrics.
3.1 Polyester Printing Application
3.1.1 Color Strength Measurements (K/S) and Analyses
Table 3 and Fig. 3 show the color intensity of screen-printed dyes applied to PE expressed as K/S, as well as the color shades of dyed fabrics. The K/S values were affected by the chemical structure and substituent of the aromatic moiety of the produced dye which produce varied print values. The color strength of 4c and 4d dyes was higher, and thus the results were better compared to 3e, 4a, and 4c dyes. This may be a result of the high coupling of the hybrid sulfonamide with the Schiff base, whose higher adsorption causes more dye and builder to disperse on the fabric. The lightness (L*) of the azo compounds 3e and 4b was lower than that of 4a and 4e, and 4c. The lower the brightness (L*) on colored fabric, the darker the color [31]. The hue angle (h°) and chroma (saturation) (c*) were calculated according to the following equations [34, 41]:
The reddish-yellowish colors of the dyes on the polyester fabric were altered by the positive values of a* and b*. The fixed temperature also helps to increase dye transfer from the printed film to the fabric by facilitating dye molecule mobility.
3.1.2 Washing Fastness
Wash fastness is known as the ability of colored fabrics to retain their color after being washed with detergents and soaps as evaluated according to ISO 105-C06 B2S technique [33]. In detail, between the two pieces that had the same diameter of undyed cotton fabric, a sample of the dyed polyester fabric was stitched. The fabric was then washed with detergent and soap at 50 °C for 30 min. The standard greyscale is used to detect staining on adjacent white fabrics and color changes in dyed samples (1: poor, 2: moderate, 3: good, 4: very good, 5: excellent).
Determination of the washing fastness properties of the prepared dyes depends on how quickly they moved out of the polyester fabric during washing. This is achieved by considering different parameters such as water solubility, the nature of the mechanical link between the dye and the fibers, the dye's molecular size, and the nature as well as the location of the charge on both the dye molecules and the fabrics.
Consequently, the polyester fabric that was printed with dispersed dyes (4d and 4c) afforded very good to excellent (4–5) staining results compared to other printed dyes (3e, 4a, and 4b) which showed moderate to very good staining (3–4). As a result of being constrained inside the polyester fabric inter-polymer chain space, 4c and 4d had bigger sizes. These acceptable results have been explained by the fact that the dye molecules possess high molecular size as well as their effective diffusion into the fabric [42, 43].
3.1.3 Perspiration Fastness
According to the perspiration fastness data (in basic and acidic solutions) proven in Table 4, it is clear that the molecular weight of the dye as well as the attraction binding force between the azo-dispersion dye and the fabric affected the dye removal from the surface of polyester fabrics [44]. From Table 3, it is clear that the polyester fabrics printed with dyes 4c and 4d showed very good to excellent perspiration fastness data (4–5), whereas the other printed dyes (3e, 4a, and 4b) exhibited moderate to very good (3–4) perspiration fastness results (Table 4).
3.1.4 Rubbing Fastness
From Table 4, the removal of loosely located or attached dyes from the surface of the printed polyester fabric in both wet and dry conditions was determined by rubbing from the surface of colored fabrics to another undyed fabric surface. The obtained results indicated that most of dyes have excellent rubbing fastness. These could be attributed to adequate dye molecule diffusion into the fabrics and might be also due to the higher dye fixation on the fiber. The greyscale (1–5) considering one being the lowest and five being the highest was used for assessment of the rubbing fastness.
3.1.5 Light Fastness
The color fastness of the prepared azo disperse dyes to light was detected using the ISO 105-B02 technique [33]. There are two factors that affect the stability of dyes in the light which are the substituent groups present in the dye molecules and the concentration of the dye molecules in the fabric. The results in Table 4 refer to that all prepared azo disperse dyes show acceptable photo-sensitivity from 6 to 7. These results could be explained by the presence of electron-donating OH and NH2 groups in dyes which might be the cause of the stability of dyes in the light.
3.1.6 Fastness to Sublimation
Table 5 displays the results for sublimation fastness of the prepared disperse dyes 3e, 4a–4d. All the prepared disperse dyes revealed good results according to the international geometric grayscale [45]. These could be explained by presence of sulfonamide groups (SO2NH2) as well as the high polarity of substituent groups [46]. Generally, the migration of azo disperse dyes from the surface of polyester fabric is related to heat treatment. Generally, the results of the sublimation fastness tests on the undyed polyester–cotton materials were typically good and matched the importance of understanding the grayscale of the current prepared disperse dyes (Table 5) [45].
3.1.7 Colony-Forming Unit (CFU) Results
Results in Table 6 and Fig. 4 revealed the antibacterial activity of fabric treated samples with 3e, 4a, 4b, 4c, and 4d as evaluated by counting technique (CFU). It has been found that fabric samples treated with 4c, 4d, and 4b had promising anti-Gram-positive activities with inhibition values (R%) of 100, 100, and 94% (respectively) against S. aureus. Whereas fabric samples treated with 4a and 3e showed moderate anti-Gram-positive activities with inhibition values of 55 and 67% (respectively) against the same test microbe. On the other hand, all treated fabrics exhibited moderate to low anti-Gram-negative activities against the test bacterium E. coli with inhibition values 53, 46, 34, 24, and 17% for compounds 4c > 4d > 4b > 4a > 3e respectively. The polyester fabric printed with dyes 3e and 4a–4d were evaluated for their antibacterial activities against the Gram-positive bacterium (S. aureus) and the Gram-negative bacterium (E. coli) using the colony-forming unit procedure as described by Gupta and Haile [39].
4 Conclusion
Printed polyester samples were improved by employing disperse azo dyes that possess sulfonamide chromophore hybrid with Schiff’s base linker. The newly developed azo-dispersion dyes showed a remarkable performance in colorimetric tests and fastness properties such as washing, perspiration, light, rubbing, and sublimation fastness when applied to silkscreen printing on polyester. The printed polyester fabrics treated with synthesized compounds, disperse dyes, that contain sulfa moieties 4-(Ncarbamimidoylsulfamoyl) phenyl) 4b, (4-(N-(5-methylisoxazol-4-yl)sulfamoyl)phenyl) 4c, and (4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl) 4d showed antibacterial activities against both Gram-positive and Gram-negative bacteria. Thus, printed fabrics treated with similar compounds could be used as industrial dyes with antibacterial activities.
References
Y.-Q. Xiao, C.-W. Kan, Coatings 12, 267 (2022)
C. Kumah, R.K. Raji, R. Pan, Autex Res. J. 20, 530 (2020)
R. Rostami, M. Zarrebini, M. Mandegari, D. Mostofinejad, S.M. Abtahi, Constr. Build. Mater. 241, 117998 (2020)
A.M. Al-Etaibi, M.A. El-Apasery, Polymers 14, 1703 (2022)
H.S. Nassar, Int. J. Text. Sci. 4, 102 (2015)
S.M. Al-Mousawi, M.A. El-Apasery, H.M. Mahmoud, Molecules 18, 7081 (2013)
S.-H. Kim, Y.-A. Son, in Dyes in Handbook of Textile and Industrial Dyeing, 1st edn., ed. by M. Clark (Woodhead Publishing, Sawston, 2011), pp.588–603
A. Abel, in Colour Design, 1st edn., ed. by J. Best (Woodhead Publishing, Sawston, 2012), pp.433–470
Y. Qian, G. Wang, G. Xiao, B. Lin, Y. Cui, Dyes Pigm. 75, 460 (2007)
H.E. Gaffer, Color. Technol. 135, 484 (2019)
M.K. Zahran, H.E.-S. Gaffer, H.M. Mashaly, Macromol. Symp. 384, 1800166 (2019)
C. Zhao, K.P. Rakesh, L. Ravidar, W.-Y. Fang, H.-L. Qin, Eur. J. Med. Chem. 162, 679 (2019)
B.K. Jadou, A.J. Hameed, A.Z. Al-Rubaie, Egypt. J. Chem. 64, 751 (2021)
N. Sahu, S. Mondal, K. Naskar, A.D. Mahapatra, S. Gupta, A.M.Z. Slawin, D. Chattopadhyay, C. Sinha, J. Mol. Struct. 1155, 152 (2018)
S.S. Ragab, A.M.K. Sweed, Z.K. Hamza, E. Shaban, A.A. El-Sayed, Fibers Polym. 23, 2114 (2022)
J. Sahoo, P. Kshiroda, N. Sarangi, S.K. Rout, A.S.K. Paidesetty, Indian J. Pharm. Sci. 82, 123 (2020)
N.M. Mallikarjuna, J. Keshavayya, J. King Saud Univ. Sci. 32, 251 (2020)
A.M. Khedr, H.A. El-Ghamry, Y.S. El-Sayed, App. Organomet. Chem. 36, e6548 (2022)
A.M. Saeed, S.S. AlNeyadi, I.M. Abdou, Heterocycl. Comm. 26, 192 (2020)
R.B. Silverman, M.W. Holladay, The Organic Chemistry of Drug Design and Drug Action, 3rd edn. (Academic Press, Boston, 2014)
S. Faro, Infect. Dis. Obstet. Gynecol. 4, 1 (1996)
E. Shaban, S.H. Nassar, S. Shabban, H.E. Gaffer, Egypt. J. Chem. 60, 73 (2017)
H.E. Gaffer, M.M.G. Fouda, M.E. Khalifa, Molecules 21, 122 (2016)
A.A. Ali, M.M. Elsawy, S.S. Salem, A.A. El-Henawy, H. Abd El-Wahab, Pigment. Resin Technol. 52, 19–32 (2021). https://doi.org/10.1108/PRT-07-2021-0078. (ahead-of-print, accessed on 12 September 2022)
H. Kaur, S.M. Lim, K. Ramasamy, M. Vasudevan, S.A.A. Shah, B. Narasimhan, Arab. J. Chem. 13, 377 (2020)
K. El-Baradie, R. El-Sharkawy, H. El-Ghamry, K. Sakai, Spectrochim. Acta A Mol. Biomol. Spectrosc. 121, 180 (2014)
H. El-Ghamry, K. Sakai, S. Masaoka, K. El-Baradie, R. Issa, J. Coord. Chem. 65, 780 (2012)
A.M. Tawfik, M.A. El-ghamry, S.M. Abu-El-Wafa, N.M. Ahmed, Spectrochi. Acta A Mol. Biomol. Spectrosc. 97, 1172 (2012)
M. I. Kiron, typical printing process | printing dyes, auxiliaries & technology, textile learner, 2013. https://textilelearner.net/typical-printing-process/. Accessed 12 Sept 2022.
S. Jose, H. Gurumallesh Prabu, L. Ammayappan, J. Nat. Fibers. 14, 40 (2017)
U. Nimkar, R. Bhajekar, Colorage 43, 135 (2006)
G.A.M. Nawwar, K.S.A. Zaher, E. Shaban, N.M.A. El-Ebiary, Fibers Polym. 21, 1293 (2020)
SIST EN ISO 105-C06:2012-Textiles-Tests for Colour Fastness-Part C06: Colour Fastness to Domestic and Commercial Laundering (ISO 105-C06:2010). https://standards.iteh.ai/catalog/standards/sist/0b4ae06f-25ab-4d0a-ab27-21fb0b32e8cc/sist-en-iso-105-c06-2012. Accessed 12 Sept 2022
ISO 105-X12:2001-Textiles-Tests for Colour Fastness-Part X12: Colour Fastness to Rubbing.: https://standards.iteh.ai/catalog/standards/iso/877d92c5-fbfe-490c-8275-7415d38cc3e8/iso-105-x12-2001. Accessed 12 Sept 2022
ISO 105-E04:2008 (Textiles-Tests for Colour Fastness-Part E04: Colour Fastness to Perspiration). https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/13/41374.html. Accessed 12 Sept 2022.
ISO 105-B02:2014(En), Textiles-Tests for Colour Fastness-Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test Available online: https://www.iso.org/obp/ui/#iso:std:iso:105:-B02:ed-6:v1:en (accessed on 12 September 2022).
EN ISO 105-P01:1995-Textiles-Tests for Colour Fastness-Part P01: Colour Fastness to Dry Heat. https://standards.iteh.ai/catalog/standards/cen/50d2e435-1a82-49fe-b512-79d62b38e5f8/en-iso-105-p01-1995. Accessed 12 Sept 2022
H.M. Abo-Salem, H.A. Abd El Salam, A. Abdel-Aziem, M.S. Abdel-Aziz, E.R. El-Sawy, Molecules 26, 4112 (2021)
D. Gupta, S.K. Khare, A. Laha, Color. Technol. 120, 167 (2004)
S.A. Abdelatef, M.T. El-Saadi, N.H. Amin, A.H. Abdelazeem, H.A. Omar, K.R.A. Abdellatif, Eur. J. Med. Chem. 150, 567 (2018)
ISO 105-B02:2013(En), Textiles-Tests for Colour Fastness-Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test. https://www.iso.org/obp/ui/#iso:std:iso:105:-B02:ed-5:v1:en. Accessed 12 Sept 2022
J.M. Jabar, T.E. Adedayo, Y.A. Odusote, Curr. Res. Green Sustain. Chem. (CRGSC) 4, 100151 (2021)
M.R. Luo (ed.), Encyclopedia of Color Science and Technology (Springer, New York, 2016), p.396
Y. Mahmoud Elkholy, M. Helmy Helal, A. Wahba Erian, Pigm. Resin Technol. 30, 168 (2001)
M. Sadeghi-Kiakhani, S. Safapour, Color. Technol. 131, 142 (2015)
S.M. Al-Mousawi, M.A. El-Apasery, M.H. Elnagdi, Molecules 18, 11033 (2013)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors, conceptualization of research topics, and formulation of specific aims; HAA, and ERE, equally performed the synthesis; MSA, evaluation of the antibacterial activity and analyzed the data; ES, prepared the printing past, measured the color as well as fastness properties and wrote the data; ERE. and MSA conceived the experiments and wrote/edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El Salam, H.A., Abdel-Aziz, M.S., El-Sawy, E.R. et al. Synthesis and Antibacterial Activity of Azo-Sulfa-Based Disperse Dyes and Their Application in Polyester Printing. Fibers Polym 24, 2751–2760 (2023). https://doi.org/10.1007/s12221-023-00255-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00255-z