Abstract
Microgravity offers an enticing synthetic knob for materials scientists to explore—however, this environment creates major challenges in hardware development that can turn a simple 3-day experiment into a 3-year long nightmare. This paper provides an overview of engineering an autoclave, compatible with NASA’s Solidification Using a Baffle in Sealed Ampoules (SUBSA) furnace, to enable microgravity hydrothermal synthesis—an acceleration-sensitive technique that processes aqueous samples above the boiling point of water. Hydrothermal synthesis is a universal chemical transformation technique that is used to produce a range of advanced materials with applications in alternative energy, healthcare, and the food industry. In this work, we use the synthesis of graphene hydrogel as a case study to verify our hardware design on Earth before launching to the International Space Station for microgravity testing. The design addresses pertinent challenges which include enabling thermal expansion while preventing air bubble formation in solution and implementing a pressure fail-safe above the maximum operating temperature. Our goal in presenting this autoclave design is to provide a step forward towards commercial-of-the-shelf microgravity hardware.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrothermal synthesis is an important method of basic chemical and material transformation that takes place in aqueous solution above the boiling point of water. (S.-H. Feng and Li 2017) This diverse synthetic technique has been widely applied in all sub-fields of chemistry, geology, and materials science research for more than a century, with the first publication of hydrothermal research reported in 1845 for the synthesis of quartz crystals using a pressure cooker. (Byrappa and Yoshimura 2001; Yang and Park 2019) Since that time, hydrothermal transformations have been a key source of technology enabling scientific advancements in nanomaterial preparation (Darr et al. 2017; Gan et al. 2020), bulk single crystal growth (Demazeau and Largeteau 2015; McMillen and Kolis 2012), and even understanding the initial chemical reactions (towards formation of amino acids and oligopeptides) leading to the origin of life on Earth. (Feng et al. 2008; S.-H. Feng and Li 2017; p. 4)
Hydrothermal transformations are inherently interesting to microgravity scientists due to their liquid medium—which makes these transformations particularly susceptible to microgravity characteristics such as the absence of buoyancy, sedimentation, and convection. (Steg 1977) However, coupled with the high interest in studying hydrothermal synthesis in microgravity, is the even higher barrier of putting theory into practice. One crucial aspect missing in the field of microgravity science research is the ability to use “commercial off-the-shelf” (COTS) hardware and research equipment to perform experiments. For example, to perform a standard hydrothermal synthesis in a typically equipped wet lab, one can simply purchase a COTS steel autoclave online and have a synthesized product in a few days. Experiments in microgravity are not that simple—as every COTS product currently on the market, for research or otherwise, is created in, and for use under, Earth’s gravity.
Herein, we describe a stainless-steel autoclave compatible with NASA’s on-orbit Solidification Using a Baffle in Sealed Ampoules (SUBSA) furnace (managed by Redwire Space Technologies, Inc.) specifically designed to enable hydrothermal synthesis in microgravity. The authors envision sharing this knowledge as a first step toward creating COTS hydrothermal reaction hardware and research equipment for use on the International Space Station National Laboratory (ISS NL) and on future private space stations orbiting our Earth. The case study described here is to hydrothermally synthesize graphene hydrogel in microgravity on the ISS. This paper will highlight both the scientific requirements (e.g., starting material, process temperature, pressure, and time) and microgravity laboratory considerations (e.g., air bubble prevention, leak-before-burst safety technology) that went into designing and building this autoclave.
Background
Hydrothermal Synthesis in Space
To the authors’ knowledge, hydrothermal synthesis has only been performed in microgravity for zeolite crystallization. Zeolites are aluminosilicate minerals that contain alkali and alkaline-earth metals. (Verma and Kaur 2021) These materials have a wide range of terrestrial applications in catalysis and as an absorbent (e.g., zeolite is a key component in commercial cat litter). Microgravity crystal growth of zeolites was first completed in 1988 on an un-manned Soviet Foton satellite (Coker et al. 1998), with many more completed as part of the CASIMIR-1 (CAtalyst Studies for Industry through MIcrogravity Research) mission (Di Renzo et al. 1994), various space shuttle missions (Coker et al. 2001; p.; Warzywoda, Baç, Jansen, et al., 2000; Warzywoda et al. 2000), and on the International Space Station (ISS). (Akata et al. 2004) The focus of these studies, as conventional, was the scientific findings related to the influence of microgravity during hydrothermal synthesis. The hardware used was generally briefly described in text and designed for compatibility using a now decommissioned furnace. The authors could not find any publicly available hardware specifications or pictures of the flight hardware mentioned in the above cited papers or in NASA technical reports. (Vlasse 1998)
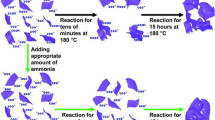
Summary of the hydrothermal synthesis of graphene hydrogel we plan to use as a case study for our microgravity autoclave. The starting material consists of an aqueous dispersion of graphene oxide flakes, which is then heated to 180 °C for 3 h for hydrothermal reduction to graphene hydrogel. When this transformation occurs on Earth, buoyancy induced effects can lead to sedimentation of the graphene flakes and subsequently a non-uniform microstructure of the resulting hydrogel. Conversely, in microgravity we anticipate Brownian motion driven effects, allowing for a homogeneous distribution of graphene flakes and a more uniform microstructure of the hydrogel sample
Graphene Aerogel Production as a Case Study
Graphene aerogel (GA) leverages graphene in a 3D-mesostructure to achieve a lightweight, high porosity, large surface area, highly conductive, low thermal conductivity material. (Gorgolis and Galiotis 2017) GA production occurs most commonly through two main steps: (1) graphene oxide (GO) flakes are suspended in an aqueous solution and hydrothermally reduced to achieve graphene hydrogel (GH); and (2) the GH is subsequently dried to remove the liquid, resulting in the final GA product. (Li et al. 2023; p. 66) The formation of GH (Fig. 1) is of main interest for our microgravity investigations—as the hydrothermal synthesis is where the graphene flakes crosslink and form the crucial 3D scaffold. During this step, the dispersion and spatial distribution of graphene sheets are influenced by agglomeration, sedimentation, and thermal gradients as it is heated and cooled. (Brück et al. 2005; Coker et al. 1998) Under Earth’s gravity, these factors can result in a GA sample with inhomogeneous dispersion of graphene flakes which can lead to anisotropic physical properties, such as, strongly anisotropic thermal conductivity. (Renteria et al. 2015) Our goal is to perform the hydrothermal synthesis of GH in microgravity in order to alleviate these influences, enabling the scaffold assembly to be primarily driven by Brownian (random thermal) motion. Synthesis in microgravity is anticipated to allow homogeneous diffusion of graphene flakes in solution, producing a more uniform 3D structure. This uniform structure is in turn anticipated to lead to isotropic physical properties, beneficial for manufacturing GA in large scale. Ultimately, this research will lay the groundwork for engineering macroscale, isotropic 3D graphene. Our payload is scheduled to launch on SpX-29 in December 2023.
SUBSA Overview
An image of the SUBSA furnace can be seen in Fig. 2a, with full furnace hardware details available elsewhere. (Spivey et al. 2003) The furnace is designed to reach a maximum process temperature of 850 °C and house a sample ampoule assembly (SAA) that can be tailored on one end to a desired experiment vessel. In this program, a stainless-steel (SS) autoclave is used as the experiment vessel. A picture of the SAA with the SS autoclave can be seen in Fig. 2b. The autoclave contains two reaction volumes where samples are grown (one sample per reaction volume). Four thermocouples (TC) are positioned along the autoclave to monitor the reaction volume temperature throughout the course of the experiment (two TCs per reaction volume). Figure 2c shows a drawing of the SAA and a close-up of the autoclave, denoting the position of the reaction volumes and TCs within the autoclave. Relevant autoclave details and dimensions will be discussed in the next section. The temperature profile of each TC is seen in Fig. 2d during a test run performed within the SUBSA ground unit, with the heater set point at 184 °C. This test run was a full representation of the flight-like experimental components, i.e., the reaction volumes contained GO dispersion and was heated for the required three hours at the elevated temperature.
Overview of the SUBSA furnace, sample ampoule assembly (SAA), and autoclave relationship. (a) Image of the SUBSA furnace and (b) SAA with the stainless-steel autoclave on the tailored end. (c) Drawing of the SAA close-up of the autoclave showing the position of the four thermocouples (TC1, TC2, TC3, and TC4) with respect to the position of Reaction Volumes A and B, shown in light blue. (d) The TC temperature profiles of a test run in the ground SUBSA unit (heater setpoint = 184 °C), heating GO dispersion in both reaction volumes for 3 h
Microgravity Autoclave Design
The autoclave was designed by Redwire Space Technologies, Inc. with input from the Stanford and Berkeley teams. Figure 3a shows a cross-section of the internal components and relevant dimensions of the autoclave. All dimensions are in mm unless otherwise noted. The autoclave was machined (Jones Machine and Tool, Fredericksburg, IN) from 316 stainless steel and cleaned and passivated before coating. The autoclave was dip-coated (Orion Industries, Chicago, IL) with fluorinated ethylene propylene (FEP) and the bottom of the piston and o-rings (i.e., areas of the headpiece in contact with the dispersion) were spray-coated with polytetrafluoroethylene (PTFE, commercial name Teflon). FEP was used for the internal autoclave coating as the dip-coating technique (not available for PTFE coating) was more suitable for the internal dimensions. The non-stick coating was used to make a highly stable and chemically nonreactive environment, thanks to the inert and hydrophobic fluorine atoms in FEP and PTFE. The coating enables the autoclave to accommodate a range of chemicals for future investigations using this hardware. (Dubecký et al. 2015; Macedonio et al. 2017; Puts et al. 2019)
Section views of the stainless-steel autoclave. (a) Cross-section of the autoclave with pertinent dimensions (mm units) and reaction volume capacity. (b) Close-up of the autoclave headpiece, highlighting its key components: the vent path, 0.1 μm frit (green), O-rings (pink), interlaced wave spring (cyan), vent grooves, and piston (yellow). (c) Pressure vs. temperature plot of the interlaced wave spring pressure (blue line) and the sample vapor pressure (red line). At the maximum process temperature (182 °C) the spring pressure is greater than the sample vapor pressure, enabling the hydrothermal synthesis to proceed. At temperature above 195 °C, the sample vapor pressure overtakes the spring pressure. This triggers the “leak-before-burst” function, allowing sample vapor to escape to prevent over-pressurizing the system. (d) A visual representation of the leak-before-burst function that occurs at temperatures above 195 °C. The sample vapor pressure (blue arrows) push against and raise the piston above the vent grooves (situated around the interior surface of the headpiece), allowing sample vapor to escape around the piston, through the spring zone (spring removed from image for clearer view), through the frit, and out the vent path. The sample vapor will eventually condense within the SUBSA furnace, preventing contamination occurring within the MSG.
The maximum external length and outer diameter (OD) of the autoclave are 97.6 mm and 16.6 mm, respectively. To achieve the desired aspect ratio of GH sample (height: diameter ∼ 2:1), and keeping in mind the maximum OD limit as well as the position of the heated zone within the SUBSA furnace, the autoclave was designed to have two reaction volumes—meaning that two GH samples will be synthesized per one autoclave. Each reaction volume is designed to hold 4.5 mL of the starting material. The reaction volumes are positioned in the center area of the autoclave to ensure the starting material is within the heated zone of the SUBSA furnace. Please refer to Fig. 2d, which verifies the process temperature within each reaction volume. Each reaction volume is coupled with a headpiece (Fig. 3b). The next two sections will describe the design of the headpieces, whose features prevent air bubbles appearing in the reaction volumes and enable a “leak-before-burst” failsafe in the event of an over-heating, over-pressurizing event.
Air Bubble Prevention
Unlike when performing a hydrothermal synthesis on Earth, where a headspace of air can be left within the autoclave, the reaction volume of an autoclave for microgravity use must be designed to be water-tight, i.e., no air bubbles allowed. If air bubbles were present, this could influence the cross-linking of GO flakes, which is a variable we do not want to parse during sample characterization. Figure 3b shows a close-up of the autoclave headpiece and its components. The piston (yellow) and interlaced wave spring (cyan) work together to help prevent the aqueous dispersion in the reaction volume from boiling and creating air bubbles. Once the GO dispersion is filled into the reaction volume, the top of the reaction volume is sealed via the piston and O-rings (pink). With known variables—the weight of the empty autoclave, the reaction volume, and density of the GO dispersion—we can ensure a water-tight seal. The spring applies the appropriate amount of pressure on the piston, which allows the necessary thermal expansion while preventing the aqueous dispersion from boiling. The plot in Fig. 3c verifies this pressure-temperature relationship by plotting the spring pressure and the dispersion’s vapor pressure with respect to temperature. The spring pressure is greater than the dispersion’s vapor pressure until 195 °C. Therefore, at the maximum process temperature (182 °C) and below, the sample will not boil.
Leak-Before-Burst
Safety is the first priority when developing an experiment to take place on the space station. To ensure pressure within the autoclave does not build beyond the process requirement, the autoclave is designed to vent sample vapor starting at 195 °C. This “leak-before-burst” feature addresses the loss of science that would occur at temperatures above 195 °C and also mitigates explosion risk in case of a furnace temperature run-away scenario. Above this temperature, the dispersion vapor pressure overtakes the spring pressure (Fig. 3c). This enables the dispersion to boil, lifting the Piston (Fig. 3d) to allow vapor (blue arrows) to escape via the Vent Grooves and out through the Vent Path. Before reaching the Vent Path, any GO brought along with the water vapor will be trapped in the 0.1 μm frit (shown in green). The remaining water vapor will be vented into the SUBSA where it will condense. The SUBSA is housed within the microgravity science glovebox (MSG) (Spivey and Flores 2008), providing another layer of security between possible experiment contamination and the interior of the space station.
Ground Verification Testing
Hydrothermal syntheses using the autoclave were tested on the SUBSA ground unit to verify the design could produce lab-quality samples. The starting material (3 mg/mL GO purchased from cheaptubes.com) was prepared at UC Berkeley and shipped to Redwire’s facility for ground testing (ramp rate = 3 °C/min; dwell time = 180 min; cool rate = 1 °C/min above, ambient cool-down below 90 °C). Before filling the reaction volumes with GO dispersion, the autoclave was thoroughly cleaned using soap and water, isopropyl alcohol bath (5 min), DI water (ultrasonic cleaning, 60 min, 35 °C), 5% acidic acid bath (10 min, 70 °C), and a DI water rinse. The autoclave was subsequently passivated per ASTM Citric III then rinsed with DI water and allowed to air dry.
The resulting GH samples were shipped back to UC Berkeley where they underwent the final drying step to GA products (Fig. 4a). Details on the full synthetic method can be found in our previous publication. (Li et al. 2023; p. 6) The GA samples produced from Reaction Volumes A and B have a density of 0.012 g/cm3 and 0.013 g/cm3, respectively. These density values are comparable to GA sample densities produced at UC Berkeley (0.015 g/cm3). Figure 4b displays Raman spectra of the GA samples produced in the UC Berkeley laboratory (black), and the test run samples produced in reaction volumes A (red) and B (green). The Raman data verifies the microgravity autoclave’s ability to produce lab-quality samples.
Conclusions
Based on ground verification, the autoclave design has proven to be a reliable vessel for producing lab-quality samples while also accounting for the unique environmental circumstances the experiment will encounter in microgravity. A key component to the autoclave design lies in the selected spring—whose spring constant dictates the position of the piston, allowing for either thermal expansion or venting of the heated sample solution. This means that for all future hydrothermal investigations within the SUBSA furnace, researchers need only to adjust the spring to a size that correlates to the density of their sample solution. This design also opens the door to solvothermal transformations (like hydrothermal synthesis, but in a non-aqueous solution) in the SUBSA furnace. The internal coating of the autoclave is the only design change that might need to be considered in a solvothermal transformation, depending on chemical reactivity within the experiment’s required temperature and time range. The flight hardware and samples were launched to the ISS on August 1, 2023 aboard NG-19 and processed by Crew-6 astronauts. The technical aspects of the autoclave operations on the ISS were a success. Subsequent analyses are underway on the returned samples.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akata, B., Yilmaz, B., Jirapongphan, S.S., Warzywoda, J., Sacco, A.: Characterization of zeolite Beta grown in microgravity. Microporous Mesoporous Mater. 71(1), 1–9 (2004). https://doi.org/10.1016/j.micromeso.2004.03.012
Brück, S., Reuβ, M., Richter, H.-E., Klein, H., Haubner, P., Ratke, L.: Gelation of hybrid aerogels in parabolic flights and on the ISS. Microgravity - Sci. Technol. 16(1), 31–34 (2005). https://doi.org/10.1007/BF02945941
Byrappa, K., Yoshimura, M.: 1—Hydrothermal Technology—Principles and Applications. In K. Byrappa & M. Yoshimura (Eds.), Handbook of Hydrothermal Technology (pp. 1–52). William Andrew Publishing. (2001). https://doi.org/10.1016/B978-081551445-9.50002-7
Coker, E.N., Jansen, J.C., Martens, J.A., Jacobs, P.A., DiRenzo, F., Fajula, F., Sacco, A.: The synthesis of zeolites under micro-gravity conditions: A review. Microporous Mesoporous Mater. 23(1), 119–136 (1998). https://doi.org/10.1016/S1387-1811(98)00046-8
Coker, E.N., Jansen, J.C., DiRenzo, F., Fajula, F., Martens, J.A., Jacobs, P.A., Sacco, A.: Zeolite ZSM-5 synthesized in space: Catalysts with reduced external surface activity. Microporous Mesoporous Mater. 46(2), 223–236 (2001). https://doi.org/10.1016/S1387-1811(01)00298-0
Darr, J.A., Zhang, J., Makwana, N.M., Weng, X.: Continuous hydrothermal synthesis of Inorganic nanoparticles: Applications and future directions. Chem. Rev. 117(17), 11125–11238 (2017). https://doi.org/10.1021/acs.chemrev.6b00417
Demazeau, G., Largeteau, A.: Hydrothermal/Solvothermal crystal growth: An Old but adaptable process. Z. Für Anorganische Und Allgemeine Chemie. 641(2), 159–163 (2015). https://doi.org/10.1002/zaac.201400515
Di Renzo, F., Fajula, F., Espiau, P., Nicolle, M.-A., Dutartre, R.: The influence of microgravity on zeolite crystallization. Zeolites. 14(4), 256–261 (1994). https://doi.org/10.1016/0144-2449(94)90093-0
Dubecký, M., Otyepková, E., Lazar, P., Karlický, F., Petr, M., Čépe, K., Banáš, P., Zbořil, R., Otyepka, M.: Reactivity of Fluorographene: A facile way toward graphene derivatives. J. Phys. Chem. Lett. 6(8), 1430–1434 (2015). https://doi.org/10.1021/acs.jpclett.5b00565
Feng, S.-H., Li, G.-H.: Chapter 4—Hydrothermal and Solvothermal Syntheses. In R. Xu & Y. Xu (Eds.), Modern Inorganic Synthetic Chemistry (Second Edition) (pp. 73–104). Elsevier. (2017). https://doi.org/10.1016/B978-0-444-63591-4.00004-5
Feng, S., Tian, G., He, C., Yuan, H., Mu, Y., Wang, Y., Wang, L.: Hydrothermal biochemistry: From formaldehyde to oligopeptides. J. Mater. Sci. 43(7), 2418–2425 (2008). https://doi.org/10.1007/s10853-007-2009-8
Gan, Y.X., Jayatissa, A.H., Yu, Z., Chen, X., Li, M.: Hydrothermal Synthesis of Nanomaterials. Journal of Nanomaterials, 2020, e8917013. (2020). https://doi.org/10.1155/2020/8917013
Gorgolis, G., Galiotis, C.: Graphene aerogels: A review. 2D Mater. 4(3), 032001 (2017). https://doi.org/10.1088/2053-1583/aa7883
Li, Z., Liu, C., Frick, J.J., Davey, A.K., Dods, M.N., Carraro, C., Senesky, D.G., Maboudian, R.: Synthesis and characterization of UiO-66-NH2 incorporated graphene aerogel composites and their utilization for absorption of organic liquids. Carbon. 201, 561–567 (2023). https://doi.org/10.1016/j.carbon.2022.09.015
Macedonio, F., Ali, A., Drioli, E.: 3.10 Membrane Distillation and Osmotic Distillation. In E. Drioli, L. Giorno, & E. Fontananova (Eds.), Comprehensive Membrane Science and Engineering (Second Edition) (pp. 282–296). Elsevier. (2017). https://doi.org/10.1016/B978-0-12-409547-2.12221-8
McMillen, C.D., Kolis, J.W.: Bulk single crystal growth from hydrothermal solutions. Phil. Mag. 92(19–21), 2686–2711 (2012). https://doi.org/10.1080/14786435.2012.685772
Puts, G.J., Crouse, P., Ameduri, B.M.: Polytetrafluoroethylene: Synthesis and characterization of the original Extreme Polymer. Chem. Rev. 119(3), 1763–1805 (2019). https://doi.org/10.1021/acs.chemrev.8b00458
Renteria, J.D., Ramirez, S., Malekpour, H., Alonso, B., Centeno, A., Zurutuza, A., Cocemasov, A.I., Nika, D.L., Balandin, A.A.: Strongly anisotropic thermal conductivity of free-standing reduced Graphene Oxide films annealed at high temperature. Adv. Funct. Mater. 25(29), 4664–4672 (2015). https://doi.org/10.1002/adfm.201501429
Spivey, R., Flores, G.: An Overview of the Microgravity Science Glovebox (MSG) Facility, and the Gravity-Dependent Phenomena Research Performed in the MSG on the International Space Station (ISS). 46th AIAA Aerospace Sciences Meeting and Exhibit. 46th AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada. (2008)., January 7 https://doi.org/10.2514/6.2008-812
Spivey, R., Gilley, S., Ostrogorsky, A., Grugel, R., Smith, G., Luz, P.: SUBSA and PFMI Transparent Furnace Systems Currently in use in the International Space Station Microgravity Science Glovebox. 41st Aerospace Sciences Meeting and Exhibit. 41st Aerospace Sciences Meeting and Exhibit, Reno, Nevada. (2003)., January 6 https://doi.org/10.2514/6.2003-1362
Steg, L. (ed.): Materials Sciences in Space with Application to Space Processing. American Institute of Aeronautics and Astronautics (1977). https://doi.org/10.2514/4.865268
Vlasse, M.: Second United States Microgravity Laboratory: One Year Report. National Aeronautics and Space Administration (1998)
Warzywoda, J., Baç, N., Jansen, J.C., Sacco, A.: Growth of zeolites a and X in low earth orbit. J. Cryst. Growth. 220(1), 140–149 (2000). https://doi.org/10.1016/S0022-0248(00)00659-X
Warzywoda, J., Baç, N., Rossetti, G.A., van der Puil, N., Jansen, J.C., van Bekkum, H., Sacco, A.: Synthesis of high-silica ZSM-5 in microgravity. Microporous Mesoporous Mater. 38(2), 423–432 (2000). https://doi.org/10.1016/S1387-1811(00)00163-3
Yang, G., Park, S.-J.: Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials. 12(7), 1177 (2019). https://doi.org/10.3390/ma12071177
Acknowledgements
Authors are thankful to G. Tipker for his help in fabricating the first iterations of the autoclave design, E. Davis for fabricating the autoclave end design, and C. Scherzer for his project management.
Funding
This work was funded by NSF Grants #1929363 (Stanford) and #1929447 (UC Berkeley) and was executed under the International Space Station U.S. National Laboratory User Agreements #UA-2019-965.
Author information
Authors and Affiliations
Contributions
J.J.F.: Writing – Original Draft, Visualization, Project Administration, Conceptualization, Methodology, Supervision, Data Curation. R.O.: Validation, Software, Resources, Visualization, Supervision, Project Administration, Data Curation, Writing – Review & Editing. Z.L., Y.O., C.L.: Conceptualization, Investigation, Validation, Writing – Review & Editing. J.M.C.: Conceptualization, Writing – Review & Editing. C.C., R.M., D.G.S.: Conceptualization, Supervision, Funding Acquisition, Writing – Review & Editing.
Corresponding authors
Ethics declarations
Ethics Approval
The authors confirm that this work is original and has not been published elsewhere nor is it currently under consideration for publication elsewhere.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frick, J.J., Ormsby, R., Li, Z. et al. Autoclave Design for Microgravity Hydrothermal Synthesis. Microgravity Sci. Technol. 36, 23 (2024). https://doi.org/10.1007/s12217-024-10109-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12217-024-10109-9