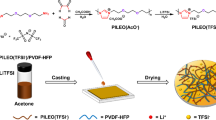
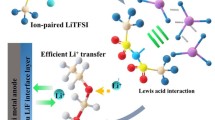
Abstract
The lithiated covalent organic framework (named TpPa-SO3Li), which was prepared by a mild chemical lithiation strategy, was introduced in poly(ethylene oxide) (PEO) to produce the composite polymer electrolytes (CPEs). Li-ion can transfer along the PEO chain or across the layer of TpPa-SO3Li within the nanochannels, resulting in a high Li-ion conductivity of 3.01 × 10−4 S/cm at 60 °C. When the CPE with 0.75 wt.% TpPa-SO3Li was used in the LiFePO4||Li solid-state battery, the cell delivered a stable capacity of 125 mA·h/g after 250 cycles at 0.5 C, 60 °C. In comparison, the cell using the CPE without TpPa-SO3Li exhibited a capacity of only 118 mA·h/g.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the demand for power sources continues to increase, various types of high-performance energy storage devices are developing rapidly [1,2,3]. Solid electrolytes are the key part of solid-state lithium-ion battery (SSLIB), which is believed as one of the next-generation battery systems with high energy density and superior safety [4]. Composite polymer electrolytes (CPEs), such as poly(ethylene oxide) (PEO), are the most promising candidate material for commercial SSLIB [5, 6]. However, the low ionic conductivity that resulted from high crystallinity still cannot be satisfied for application. To solve this problem, inorganic particles such as oxides and sulfides were widely used as fillers to decrease the crystallinity of PEO or construct extra Li-ion conductive pathways [7, 8]. Traditional efficient fillers have some shortcomings: (1) oxides usually need a high-temperature treatment, such as Li7La3Zr2O12 (> 900 °C) [9]; (2) most oxides and sulfides are sensitive to water and air [10, 11]; (3) the high weight content of fillers hinders the improvement in the overall battery’s energy density. Therefore, great efforts in finding better fillers are needed for practical applications.
Covalent organic frameworks (COFs) are a new kind of porous materials with advantages such as highly adjustable structures, light mass, high specific surface area, and unique one-dimensional channel structures [12, 13]. Although these features are suitable for use as fillers, COFs are rarely applied in CPEs due to their poor ionic conductivity [14,15,16]. To enhance the intrinsic Li-ion conductivity of COFs, this study prepared a lithiated COF (named TpPa-SO3Li) by a mild chemical lithiation strategy. The obtained TpPa-SO3Li was selected as the active filler in the CPE for SSLIB. Compared with other fillers without Li-ion conductivity, the TpPa-SO3Li provides a rapid Li-ion transfer via the –SO3Li groups as Li-ion conducting sites in the nanochannels, which work as the expressway between the PEO chains, leading to enhanced Li-ion conductivity. Moreover, the extra advantages of high chemical stability and low density provide the potential for practical application. Results show that the TpPa-SO3Li serves as promising filler in CPE and enhances the electrochemical performances of LiFePO4||Li SSLIB.
Experiment Section
TpPa-SO3Li was prepared by solvothermal and chemical lithiation methods [13]. TpPa-SO3Li, PEO (molecular weight Mw ≈ 6 × 105), and LiClO4 were dissolved in anhydrous acetonitrile with a certain weight ratio of EO: Li = 18:1. The homogenized suspension was cast into a polytetrafluoroethylene mold and dried at room temperature for 24 h. After further drying at 55 °C for another 12 h under vacuum, the CPE membrane (named (PEO)18LiClO4/TpPa-SO3Li(x), x is the mass fraction of TpPa-SO3Li) was obtained. The CPE without TpPa-SO3Li was denoted as (PEO)18LiClO4.
Results and Discussions
Figure 1a shows the chemical structure of TpPa-SO3H, which was synthesized by the Schiff base condensation reaction of 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde (Tp) and 2,5-diaminobenzenesulfonic acid (Pa-SO3H). The enol-imine form converted to keto-enamine form after condensation. It was lithiated by Lewis acid–base reaction using lithium acetate. As shown in Fig. 1b, the Fourier transform infrared (FT-IR) peaks of TpPa-SO3H at 1240, 1575, and 3400 cm−1 represent the C–N, C = C, and enamine N–H bonds, respectively. The peak at 3200 cm−1, which corresponds to O–H bonds in sulfonates, is found in TpPa-SO3H. After being lithiated, this peak significantly weakened, indicating that the H atoms in the –OH groups are replaced by Li atoms. Meanwhile, the O–Li bonds form and show a characteristic peak at 1750 cm−1, which overlaps with the characteristic peak of C=O bonds. X-ray photoelectron spectroscopy (XPS) spectra (Fig. 1c and Fig. S1) also confirm the lithium substitution. Due to the splitting of the spin–orbit, double peaks appear in the S 2p spectrum and shift to higher binding energy after being lithiated. It confirms that the environment of the S atom has changed, which is attributed to the substitution of H atoms in the sulfonates by Li atoms [17].
Scanning electron microscopy (SEM) images show that the TpPa-SO3H and TpPa-SO3Li have similar stacked particle structures (Fig. 1d, e). These particles have an average diameter of less than 100 nm and are formed by stacking porous nanosheets. The X-ray diffraction (XRD) pattern of TpPa-SO3H (Fig. 1f) shows the characteristic peaks at 2θ = 4.6° and 26.2° that correspond to (100) and (001) planes, respectively. This confirms the ordered pores with a pore size of 1.88 nm and an interlayer distance of 3.4 Å. This indicates that the lithiation process has basically no effect on the layered structure and morphology. The crystalline structure of TpPa-SO3Li can be verified by the transmission electron microscopy (TEM) image (Fig. S2) as well. The Li content obtained by the inductively coupled plasma optical emission spectrometer is about 1.53 wt.%, which is a little lower than the theoretical value of 2.33 wt.%.
TpPa-SO3Li can be uniformly dispersed in the casting solution without sedimentation (Fig. S3). As shown in Fig. 2a and Fig. S4, the CPEs are flexible with a thickness of ~ 50 μm and have a uniform surface without visible defects or pellets (Fig. 2b, c). In addition, there is no obvious difference in the surface morphology of CPEs with or without TpPa-SO3Li. The uniform dispersion of TpPa-SO3Li in PEO can be demonstrated by the cross-sectional energy-dispersive spectrometer (EDS) mapping of S (Fig. 2d). The XRD characterization was performed to study the crystallinity of CPEs in Fig. 2e, indicating that the introduction of TpPa-SO3Li with various ratios has no effect on the crystallinity of CPEs either [5]. After adding 0.75 wt.% TpPa-SO3Li, the melting enthalpy of CPEs changes from 88.03 J/g to 91.88 J/g. (Fig. 2f). Correspondingly, the crystallinity of CPEs increased from 41.19 to 42.99%, which suggests that the crystallinity of CPEs is hardly affected.
a Digital photographs of (PEO)18LiClO4/TpPa-SO3Li(0.75). SEM images of (b) (PEO)18LiClO4 and (c) (PEO)18LiClO4/TpPa-SO3Li(0.75). d Cross-sectional SEM image and S EDS mapping of (PEO)18LiClO4/TpPa-SO3Li(0.75). e XRD patterns of the CPEs. f DSC traces of (PEO)18LiClO4 and (PEO)18LiClO4/TpPa-SO3Li(0.75)
To study the influence of TpPa-SO3Li on the ionic conductivity, this work assembled SS|CPE|SS cells (SS represents stainless steel) and performed electrochemical impedance spectroscopy (EIS). Figure 3a shows the typical spectra of (PEO)18LiClO4/TpPa-SO3Li(0.75) at different temperatures. The resistance decreases rapidly with the increase in temperature. At 50 °C and above, the semicircle disappears, indicating a decreased interface resistance because of the improved interface interaction. As shown in Fig. 3b, with the increase in the TpPa-SO3Li content, the ionic conductivity shows a trend of initially rising and then falling with inflection points at 0.75 wt.% TpPa-SO3Li content. Taking the curve at 60 °C as an example, the ionic conductivity ranges from 1.62 × 10−4 to 3.01 × 10−4 S/cm as the filling amount increases from 0 to 0.75 wt.%. When the content is further increased and exceeds the threshold, the conductivity drops rapidly to even lower than that of (PEO)18LiClO4. This phenomenon can be explained by the percolation behavior. It is attributed to aggregation, phase separation, and bubbles caused by excess TpPa-SO3Li, which goes against ion conduction. Moreover, due to the low density and high specific surface area of TpPa-SO3Li, the ionic conductivity can be improved at a very low content, and the threshold of the percolation behavior is only about 0.75 wt.%.
a EIS spectra of (PEO)18LiClO4/TpPa-SO3Li(0.75) at different temperatures. b Ionic conductivity of CPEs at different temperatures and TpPa-SO3Li contents. c Arrhenius plot for the ionic conductivity of CPEs with TpPa-SO3H or TpPa-SO3Li. d EIS spectra of (PEO)18LiClO4/TpPa-SO3Li(0.75) before and after polarization (inset shows the corresponding I-t curve). e Schematic illustration of Li-ion conductive mechanism of (PEO)18LiClO4/TpPa-SO3Li(0.75)
To study the effect of the lithiation sites, (PEO)18LiClO4/TpPa-SO3H(0.75) was prepared with the same method, and its ionic conductivity was tested at different temperatures. As shown in Fig. 3c, the ionic conductivity of (PEO)18LiClO4/TpPa-SO3H(0.75) is significantly lower than that of (PEO)18LiClO4. Since TpPa-SO3H does not have Li-ion conductivity, its uniform dispersion in CPEs may form micro-domains that are not conducive to the Li-ion. In addition, the H-ions that dissociated from the sulfonates hinder the Li-ions from entering the nanochannels of TpPa-SO3H by steric hindrance and Coulomb interaction, which further increases the Li-ion diffusion energy barrier. The chemical lithiation method can replace H-ions with Li-ions, introduce evenly distributed Li-ion jumping conduction sites, and construct low-resistance transmission channels.
In addition to the high ionic conductivity, sulfonates in TpPa-SO3Li serve as single Li-ion conduction sites, which is beneficial to increase the Li-ion transference number \((t_{\text{Li}^{+}})\) [13]. Chronoamperometry and EIS were carried out at 60 °C to measure the \(t_{\text{Li}^{+}}\) using Li|CPE|Li cells. As shown in Fig. 3d and Fig. S5, (PEO)18LiClO4/TpPa-SO3Li(0.75) delivers a \(t_{\text{Li}^{+}}\) of 0.300, while (PEO)18LiClO4 shows a \(t_{\text{Li}^{+}}\) of 0.258.
The improvements in the ionic conductivity and \(t_{\text{Li}^{+}}\) benefit from the structure and composition of TpPa-SO3Li. As shown in Fig. 3e, the unique one-dimensional channel structure provides rapid Li-ion transfer and shortens the transfer distance. Meanwhile, lithiation sites in the channels significantly reduce the Li-ion diffusion barrier.
The linear sweep voltammetry (LSV) measurement was performed at 60 °C to study the electrochemical stability window of the CPEs (Fig. 4a). The result shows that (PEO)18LiClO4 and (PEO)18LiClO4/TpPa-SO3Li(0.75) are electrochemically stable at the voltage range of 1.0–4.5 V. Within the operating voltage range of LiFePO4, both PEO and TpPa-SO3Li can be kept stable.
a LSV profiles of (PEO)18LiClO4 and (PEO)18LiClO4/TpPa-SO3Li(0.75). b Charge/discharge curves of LiFePO4|(PEO)18LiClO4|Li. c Charge/discharge curves of LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li. d Cycling performance of LiFePO4|CPE|Li cells at 0.5 C and 60 °C. e EIS spectra of LiFePO4|(PEO)18LiClO4|Li. f EIS spectra of LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li
Li symmetric cells were characterized by charge/discharge measurements at 60 °C, 0.1 mA/cm2, and 0.1 mA·h/cm2 to study the interfacial stability. As shown in Fig. S6, the overpotential of Li|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li is much smaller than that of Li|(PEO)18LiClO4|Li. Moreover, Li|(PEO)18LiClO4|Li is short-circuited after cycling for 80 h, which signifies that (PEO)18LiClO4/TpPa-SO3Li(0.75) has a more stable interface with lithium than (PEO)18LiClO4.
To explore the practical application of the CPEs, this work assembled LiFePO4|CPE|Li cells and measured their electrochemical performance at 60 °C and 0.5 C with activation at 0.1 C for five cycles. As shown in Fig. 4b, the polarization voltage of LiFePO4|(PEO)18LiClO4|Li significantly increases with the cycle process, while LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li exhibits a stable polarization voltage of 0.11 V (Fig. 4c). This proves that it has the potential to be applied in SSLIB.
As shown in Fig. 4d, LiFePO4|(PEO)18LiClO4|Li delivers initial and stable discharge capacities of 151.2 and 118 mA·h/g in the first 250 cycles, respectively. On the other hand, LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li shows initial and stable discharge capacities of 154.7 and 125 mA·h/g, respectively. The unstable Coulombic efficiency may be due to the side reaction caused by the trace solvent in the CPEs. The Coulombic efficiency of LiFePO4|(PEO)18LiClO4|Li was basically stable after 75 cycles. The unlithiated sulfonate in TpPa-SO3Li will be electrochemically lithiated, leading to a fluctuating Coulombic efficiency of LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li. To test the stability of LiFePO4|CPE|Li cells to temperature, the cells were cooled to room temperature for 24 h after 250 cycles and then tested at 60 °C and 0.5 C again (Fig. S7). The stable discharge capacities of LiFePO4|(PEO)18LiClO4|Li and LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li decayed to 100 and 113 mA·h/g, respectively. The corresponding capacity retention rates before and after cooling were 84.7% and 90.4%, respectively, indicating that LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li was more stable to temperature.
EIS spectra at the reduction state were tested to study the interfacial stability. As shown in Fig. 4e, LiFePO4|(PEO)18LiClO4|Li shows two overlapped semicircles in the middle- and high-frequency range. Moreover, the impedance increases rapidly during cycling, which means a serious interface reaction between (PEO)18LiClO4 and electrodes. After three cycles, LiFePO4|(PEO)18LiClO4/TpPa-SO3Li(0.75)|Li shows only one semicircle (Fig. 4f), and the corresponding impedance is smaller than that of the initial state. Therefore, the addition of TpPa-SO3Li effectively improved the interface stability between the CPE and electrodes.
Conclusions
The TpPa-SO3Li was prepared via a mild chemical lithiation strategy and applied as active filler in the PEO-based CPE. The –SO3Li groups in TpPa-SO3Li work as Li-ion conducting sites in the nanochannels, providing an expressway between the PEO chains. Therefore, high ionic conductivity of 3.01 × 10−4 S/cm was obtained at 60 °C. When the CPE with only 0.75 wt.% TpPa-SO3Li was assembled in the SSLIB, it delivered a reversible capacity of 125 mA·h/g after 250 cycles at 0.5 C and 60 °C. This strategy provides a convenient method to prepare Li-ion conducting COFs and provides a new path for lightweight CPEs.
References
Li C, Zhang X, Lv Z et al (2021) Scalable combustion synthesis of graphene-welded activated carbon for high-performance supercapacitors. Chem Eng J. 414:128781
Yi S, Wang L, Zhang X et al (2021) Cationic intermediates assisted self-assembly two-dimensional Ti3C2Tx/rGO hybrid nanoflakes for advanced lithium-ion capacitors. Sci Bull 66(9):914–924
Yu Z, Zhang JJ, Wang C et al (2020) Flame-retardant concentrated electrolyte enabling a LiF-rich solid electrolyte interface to improve cycle performance of wide-temperature lithium-sulfur batteries. J Energy Chem 51:154–160
Zhou Q, Ma J, Dong SM et al (2019) Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv Mater 31(50):1902029
Ito Y, Kanehori K, Miyauchi K et al (1987) Ionic conductivity of electrolytes formed from PEO-LiCF3SO3 complex low molecular weight poly(ethylene glycol). J Mater Sci 22(5):1845–1849
Zhu XQ, Wang K, Xu YN et al (2021) Strategies to boost ionic conductivity and interface compatibility of inorganic-organic solid composite electrolytes. Energy Storage Mater 36:291–308
Boaretto N, Meabe L, Martinez-Ibañez M et al (2020) Review–polymer electrolytes for rechargeable batteries: from nanocomposite to nanohybrid. J Electrochem Soc 167(7):070524
Wang JJ, Yuan B, Pan FS et al (2020) Nano-silica-decorated poly(m-phenylene isophthalamide) separator with enhanced mechanical and electrolyte wetting properties for lithium-ion batteries. Trans Tianjin Univ 26(4):256–264
Murugan R, Thangadurai V, Weppner W (2007) Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed 46(41):7778–7781
Hakari T, Nagao M, Hayashi A et al (2015) All-solid-state lithium batteries with Li3PS4 glass as active material. J Power Sources 293:721–725
Xu XY, Li YY, Cheng J et al (2020) Composite solid electrolyte of Na3PS4-PEO for all-solid-state SnS2/Na batteries with excellent interfacial compatibility between electrolyte and Na metal. J Energy Chem 41:73–78
Cao Y, Liu C, Wang MD et al (2020) Lithiation of covalent organic framework nanosheets facilitating lithium-ion transport in lithium-sulfur batteries. Energy Storage Mater 29:207–215
Jeong K, Park S, Jung GY et al (2019) Solvent-free, single lithium-ion conducting covalent organic frameworks. J Am Chem Soc 141(14):5880–5885
Sun W, Zhang J, Xie M et al (2020) Ultrathin aramid/COF heterolayered membrane for solid-state Li-metal batteries. Nano Lett 20(11):8120–8126
Zhang G, Hong YL, Nishiyama Y et al (2019) Accumulation of glassy poly(ethylene oxide) anchored in a covalent organic framework as a solid-state Li+ electrolyte. J Am Chem Soc 141(3):1227–1234
Guo ZB, Zhang YY, Dong Y et al (2019) Fast ion transport pathway provided by polyethylene glycol confined in covalent organic frameworks. J Am Chem Soc 141(5):1923–1927
Wang SH, Yue J, Dong W et al (2019) Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes. Nat Commun 10(1):4930
Acknowledgements
This work was supported by the State Key Laboratory of Catalytic Materials and Reaction Engineering (RIPP, SINOPEC), the National Natural Science Foundation of China (Nos. 21878216, 22005215), Hebei Province Innovation Ability Promotion Project (No. 20312201D), and the National Key Research and Development Program of China (No. 2019YFE0118800).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, Y., Cao, Y., Li, G. et al. Enhanced Electrochemical Performance of Poly(ethylene oxide) Composite Polymer Electrolyte via Incorporating Lithiated Covalent Organic Framework. Trans. Tianjin Univ. 28, 67–72 (2022). https://doi.org/10.1007/s12209-021-00300-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-021-00300-z