Abstract
Impairments of hearing and balance are major problems in the field of occupational and environmental health. Such impairments have previously been reported to be caused by genetic and environmental factors. However, their mechanisms have not been fully clarified. On the other hand, the inner ear contains spiral ganglion neurons (SGNs) in the organ of Corti, which serve as the primary carriers of auditory information from sensory cells to the auditory cortex in the cerebrum. Inner ears also contain a vestibule in the vicinity of the organ of Corti—one of the organs responsible for balance. Thus, inner ears could be a good target to clarify the pathogeneses of sensorineural hearing losses and impaired balance. In our previous studies with c-Ret knock-in mice and Endothelin receptor B (Ednrb) knock-out mice, it was found that syndromic hearing losses involved postnatal neurodegeneration of SGNs caused by impairments of c-Ret and Ednrb, which play important roles in neuronal development and maintenance of the enteric nervous system. The organ of Corti and the vestibule in inner ears also suffer from degeneration caused by environmental stresses including noise and heavy metals, resulting in impairments of hearing and balance. In this review, we introduce impairments of hearing and balance caused by genetic and environmental factors and focus on impairments of SGNs and the vestibule in inner ears as the pathogeneses caused by these factors.
Similar content being viewed by others
Introduction
It has been reported that about 250 million people worldwide suffer from hearing losses. About 30 % of people with congenital hearing loss are syndromic and the remaining 70 % are non-syndromic. In addition, most elderly people develop age-related (late-onset) hearing loss [1–3]. In general, these hearing losses have been classified as different diseases due to distinct pathogeneses [1, 2]. Sensorineural hearing losses are caused by impairments of inner ears and are difficult to cure due to the location and complex morphology of inner ears [1, 2]. Sensorineural hearing loss is a clinically heterogeneous disease leading to negative impacts on quality of life (QOL) in all generations. Sensorineural hearing loss involves different onset, severity and pathological sites.
Inner ears have been analyzed in order to clarify the pathogeneses of sensorineural hearing losses. The inner ears contain the organ of Corti and stria vascularis (SV). The SV is essential for maintenance of endolymph potential. The organ of Corti contains two kinds of sensory cells [inner hair cells (IHCs) and outer hair cells (OHCs)] and plays an important role in mechanotransduction, by which sound stimuli are converted into electric stimuli. Auditory information from the sensory cells is transferred to spiral ganglion neurons (SGNs) as the primary carriers and is eventually transferred to the auditory cortex in the cerebrum [1, 2]. The SV consists of marginal cells, melanocytes (also known as intermediate cells) and basal cells, and has been shown to maintain high levels of potassium ion for endocochlear potential (EP) [4, 5]. Melanocytes in the inner ear are located specifically in the SV, and defects in melanocytes lead to impaired EP levels resulting in hearing loss [6]. Thus, disturbance of these constituent cells in inner ears has been shown to cause hearing losses [7]. Inner ears also contain a vestibule in the vicinity of the organ of Corti. Vestibular hair cells covered with otoconia play an important role in mechanotransduction, by which gravity impulses are converted into neural impulses. Impairments of vestibular hair cells have been shown to cause abnormal behaviors including balance [8]. Thus, the vestibule containing hair cells and an otolith is one of the organs responsible for balance.
Impairments of hearing and balance—both major problems in the field of occupational and environmental health—are caused by the intricate interplay of genetic, aging and environmental factors [1–3]. However, there is limited information about the pathogenesis of hearing loss and imbalance. This review focuses on hearing impairments caused by neurodegeneration of SGNs due to impairments of hearing-related genes (c-Ret and Ednrb) and by environmental stresses [low frequency noise (LFN) and heavy metals].
c-Ret-mediated hearing losses
c-RET is a receptor-tyrosine kinase [9]. Glial cell line-derived neurotrophic factor (GDNF)—one of the ligands for c-RET—exerts its effect on target cells by binding to a glycosyl phosphatidylinositol (GPI)-anchored cell surface protein (GFRα1). This binding facilitates the formation of a complex with the receptor tyrosine kinase c-RET. Formation of this complex activates c-RET autophosphorylation as a trigger for c-RET-mediated signaling pathways to give positive signals for cell survival [9–12]. Previous studies have also indicated that GDNF stimulates a Ret-independent signaling pathway [10, 13, 14]. Tyrosine 1062 (Y1062) in c-Ret plays an important role in kinase activation as one of the autophosphorylation sites, and is also a multi-docking site for several signaling molecules including SHC, a transmitter for c-Ret-mediated signaling pathways [13, 15, 16]. In both mice and humans, c-RET has been shown to be essential for the development and maintenance of the enteric nervous system (ENS) [13, 15] and to be the most frequent causal gene of Hirschsprung disease (HSCR; megacolon disease) (in 20–25 % of cases) in humans [17, 18]. In fact, severe HSCR (e.g., total intestinal agangliosis and impaired development of the kidney) has been shown to develop in homozygous knock-in mice in which Y1062 in c-Ret was replaced with phenylalanine (c-Ret-KIY1062F/Y1062F-mice), while heterozygous c-Ret Y1062F knock-in mice (c-Ret-KIY1062F/+-mice) are reported to have no HSCR-linked phenotypes [11]. Thus, the results of previous studies indicate that HSCR in mice develops recessively [11], while HSCR in humans has been shown to develop dominantly due to RET mutations [19]. As described above, c-Ret and c-RET are crucial genes for HSCR; however, there had been no direct evidence to link c-Ret and c-RET to hearing impairments in mice or humans. Our recent studies have shown that complete unphosphorylated Y1062 in c-Ret, with no change in expression level, caused congenital hearing loss in c-Ret-KIY1062F/Y1062F-mice [20], while partially unphosphorylated c-Ret led to normal hearing development until 1 month of age but then accelerated age-related hearing loss in c-Ret-KIY1062F/+-mice [21]. Thus, impairments of c-Ret phosphorylation monogenetically result in early-onset syndromic hearing loss as well as late-onset non-syndromic hearing loss. Our results correspond in part to the results of previous studies demonstrating that c-Ret, GFRα1 and GDNF are expressed in auditory neurons [22, 23] and that GDNF has a protective effect on antibiotic-mediated ototoxicities [24–27].
Ednrb-mediated hearing loss
Waardenburg-Shah syndrome (WS type IV, WS-IV), which is caused by mutations in the transcription factor Sox10 [28], cytokine endothelin (ET)-3 [29] and its receptor endothelin receptor B (Ednrb) [30], is characterized by hypopigmentation, megacolon disease and hearing loss. The incidence of WS is 1 per 10,000 to 20,000 people [31]. Endothelin receptor B (Ednrb/EDNRB) belongs to the G-protein-coupled receptor family that mediates the multifaceted actions of endothelins [32, 33]. Mutations of Ednrb/EDNRB have been shown to cause embryonic defects in melanocytes and enteric ganglion neurons derived from the neural crest, resulting in hypopigmentation, megacolon disease and congenital hearing loss. In previous studies with animal models, both piebald-lethal rats in which Ednrb is spontaneously mutated [34] and Ednrb homozygous knock-out [Ednrb(−/−)] mice [35] have been shown to have typical WS-IV phenotypes. Thus, previous studies indicate that Ednrb is a key regulatory molecule for embryonic development of melanocytes and peripheral neurons, including neurons in the ENS. Previous studies also demonstrated that impairments of Ednrb/EDNRB cause syndromic hearing loss due to congenital defects of melanocytes in the stria vascularis of the inner ear [30, 32–35]. In our previous study, Ednrb protein was expressed in SGNs from wild-type (WT)-mice on postnatal day 19 (P19), while it was undetectable in SGNs from WT-mice on P3. Correspondingly, Ednrb homozygously deleted mice [Ednrb(−/−)-mice] developed congenital hearing loss (Fig. 1) [36]. Thus, expression of Ednrb expressed in SGNs in the inner ears is required for postnatal development of hearing in mice. A therapeutic strategy for congenital hearing loss in WS-IV patients has not been established. EDNRB expressed in SGNs could be a novel potential therapeutic strategy for congenital hearing loss in WS-IV patients.
Schematic summary of congenital deafness caused by neurodegeneration of spiral ganglion neurons (SGNs) in c-Ret-knock-in-mice and Ednrb-knock-out-mice. The x-axis indicates age (days after birth) of mice. Triangles Rosenthal’s canals in wild-type (WT) (light gray background), or homozygous c-Ret-knock-inY1062F/Y1062F (Ret-KI) [20] and homozygous Ednrb-knock-out-mice (Ednrb-KO) (white background) [36]; gray circles/no outline immature SGNs; gray circles/thin outline SGNs; gray circles/bold outline SGNs with “phosphorylated Y1062 in c-Ret” or “expression of Ednrb”. Dark gray circles/dotted outline SGNs with “decreased phosphorylation of Y1062 in c-Ret” or “decreased expression of Ednrb”. a c-Ret-KI- and Ednrb-KO-mice suffer from congenital deafness with neurodegeneration of SGNs. b c-Ret-KIY1062F/Y1062F-mice showed no Y1062-phosphorylated SGNs even on P8, although Y1062-phosphorylated SGNs began to appear in WT mice from P8 [20]. Ednrb-KO-mice also showed undetectably low expression level of Ednrb in SGNs on P8, although Ednrb-positive SGNs began to appear in WT mice from P8 [36]
Neurodegeneration of SGNs caused by impairments of c-Ret and Ednrb
Phosphorylation of Y1062 in c-Ret has been shown to mediate several biological responses, including development and survival of neuronal cells [13, 37]. In our recent studies, c-Ret-KIY1062F/Y1062F-mice developed severe congenital deafness with neurodegeneration of SGNs on postnatal day (P) 8-18, while c-Ret-KIY1062F/Y1062F-mice showed morphology of SGNs comparable to that in WT mice on P2-3 [20]. Phoshorylation of Y1062 in c-Ret of SGNs from WT mice on P2-3 was below the limit of detection, while that on P8-18 was clearly detectable [20]. Thus, it is thought that SGNs from c-Ret-KIY1062F/Y1062F-mice developed normally at least until P3 after birth, when Y1062 in c-Ret of SGNs from WT mice is unphospholylated. However, in c-Ret-KIY1062F/Y1062F-mice, phosphorylation of Y1062 is no longer maintained by P8–P18, when Y1062 in c-Ret of SGNs from WT mice exhibits significant phosphorylation [20]. Furthermore, partially unphosphorylated Y1062 in c-Ret of SGNs accelerated age-related hearing loss with accelerated reduction of SGNs from 4 months of age, while normal hearing and normal density of SGNs were observed at least until 1 month of age, when hearing has matured [21]. On the other hand, Ednrb protein was expressed in SGNs from WT-mice on postnatal day 19 (P19), while it was undetectable in SGNs from WT-mice on P3. Correspondingly, Ednrb(−/−)-mice with congenital hearing loss showed a decreased number of SGNs (Fig. 1) and degeneration of SGNs on P19 but not on P3 [36]. Thus, our results show that Ednrb expression in SGNs in inner ears is required for postnatal survival of SGNs in mice. The neurodegeneration of SGNs from c-Ret-KIY1062F/Y1062F-mice and Ednrb(−/−)-mice did not show typical apoptotic signals and did not involve disturbance of hair bundles of IHCs and OHCs [20, 36]. The congenital hearing loss involving neurodegeneration of SGNs as well as megacolon disease in Ednrb(−/−)-mice were improved markedly by introducing an Ednrb transgene under the control of the dopamine beta-hydroxylase promoter (Ednrb(−/−); DBH-Ednrb-mice). Neurodegeneration of SGNs was restored by introducing constitutively activated RET also in the case of c-Ret-mediated hearing loss. Thus, our results indicate that c-RET and EDNRB expressed in SGNs could be molecular targets in the prevention of hearing impairments.
Environmental stress-related impairments of hearing and balance
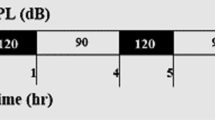
Exposure to noise is recognized as one of the major environmental factors causing hearing loss [1]. Noise consists of sound with broad frequencies, but there is limited information about the frequency-dependent influence of noise on health. Low frequency noise (LFN) is constantly generated from natural and artificial sources. The frequency range of LFN is usually defined as being below 100 Hz, while that of infrasound is usually below 20 Hz [38]. In our recent study, we found that chronic exposure to LFN at moderate levels of 70 dB sound pressure level (SPL) causes impaired balance involving morphological abnormalities of the vestibule with increased levels of oxidative stress (Fig. 2) [39]. Previous studies have shown that behavioral impairments induced by antibiotics involved degeneration of vestibular cells and oxidative stress [40, 41]. In addition, a previous study has shown that antioxidant compounds prevent noise-induced hearing loss [42]. Ototoxicity caused by oxidative stress in inner ears has been shown to accompany impairment of antioxidant enzymes [42]. Thus, existing studies indicate the necessity for further investigation of a causal molecule related to oxidative stress in vestibular hair cells affected by LFN, and of the preventive effect of antioxidants on impaired balance caused by LFN exposure. On the other hand, exposure to heavy metals including mercury, cadmium and arsenic has been suggested to cause impairments in balance [43] and hearing [44–46] in humans and experimental animals. Smoking has also been shown to affect hearing in humans [47]. In previous studies, childhood exposure to heavy metals has been shown to sensitively affect hearing development in humans [48–50]. Aging has also been shown to affect sensitivities to ototoxic factors in mice [51]. Therefore, further studies are needed to determine the age-specific susceptibilities to environmental stresses, including heavy metals, in terms of ototoxicity in mice and humans.
Schematic summary of impaired balance in mice caused by exposure to low frequency noise (LFN). Chronic exposure to low frequency noise (LFN, 0.1 kHz) at moderate levels of 70 dB sound pressure level (SPL) causes impaired balance involving morphological impairments of the vestibule with enhanced levels of oxidative stress [39]
Conclusions
Our studies provide direct evidence that c-RET and EDNRB expressed in SGNs are novel targets for hearing loss. These studies underline the importance of considering the activity as well as the expression of the target molecule in order to elucidate the etiologies of hereditary deafness. In addition, environmental stresses, including exposure to noise and heavy metals, can cause impairments of hearing and balance that are affected intricately by aging and genetic factors. Information obtained in previous studies prompts further investigation of the influence of environmental stresses on the impairment of hearing and balance with consideration of aging and genetic factors to develop new diagnostic, preventive and therapeutic strategies against impairment of hearing and balance.
References
Lalwani AK, Gürtler N. Sensorineural hearing loss, the aging inner ear, and hereditary hearing impairment. In: Lalwani AK, editor. CURRENT diagnosis & treatment in otolaryngology—head & neck surgery. 2nd edn. New York: McGraw-Hill; 2008. pp. 683–704.
Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet. 2008;9:277–90.
Gratton MA, Vázquez AE. Age-related hearing loss: current research. Curr Opin Otolaryngol Head Neck Surg. 2003;11:367–71.
Salt AN, Melichar I, Thalmann R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope. 1987;97:984–91.
Steel KP, Barkway C. Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development. 1989;107:453–63.
Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci USA. 2008;105:1751–6.
Gürtler N, Lalwani AK. Etiology of syndromic and nonsyndromic sensorineural hearing loss. Otolaryngol Clin North Am. 2002;35:891–908.
Zhao X, Jones SM, Yamoah EN, Lundberg YW. Otoconin-90 deletion leads to imbalance but normal hearing: a comparison with other otoconia mutants. Neuroscience. 2008;153:289–99.
Kato M, Iwashita T, Takeda K, Akhand AA, Liu W, Yoshihara M, et al. Ultraviolet light induces redox reaction-mediated dimerization and superactivation of oncogenic Ret tyrosine kinases. Mol Biol Cell. 2000;11:93–101.
Trupp M, Scott R, Whittemore SR, Ibáñez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274:20885–94.
Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, et al. A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol. 2004;24:8026–36.
Drosten M, Pützer BM. Mechanisms of disease: cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nat Clin Pract Oncol. 2006;3:564–74.
Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94.
Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, et al. GDNF triggers a novel ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor alpha1. FEBS Lett. 1999;463:63–6.
Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–79.
Kato M, Takeda K, Kawamoto Y, Iwashita T, Akhand AA, Senga T, et al. Repair by Src kinase of function-impaired RET with multiple endocrine neoplasia type 2A mutation with substitutions of tyrosines in the COOH-terminal kinase domain for phenylalanine. Cancer Res. 2002;62:2414–22.
Moore SW, Johnson AG. Hirschsprung’s disease: genetic and functional associations of Down’s and Waardenburg syndromes. Semin Pediatr Surg. 1998;7:156–61.
Moore SW. The contribution of associated congenital anomalies in understanding Hirschsprung’s disease. Pediatr Surg Int. 2006;22:305–15.
Inoue K, Shimotake T, Iwai N. Mutational analysis of RET/GDNF/NTN genes in children with total colonic aganglionosis with small bowel involvement. Am J Med Genet. 2000;93:278–84.
Ohgami N, Ida M, Shimotake T, Sakashita N, Sone M, Nakashima T, et al. c-Ret-mediated hearing loss in mice with Hirschsprung disease. Proc Natl Acad Sci USA. 2010;107:13051–6.
Ohgami N, Ida-Eto M, Sakashita N, Sone M, Nakashima N, Tabuchi K, et al. Partial impairment of c-Ret at tyrosine 1062 accelerates age-related hearing loss in mice. Neurobiol Aging. 2012;33:626.e25–34.
Stöver T, Gong TL, Cho Y, Altschuler RA, Lomax MI. Expression of the GDNF family members and their receptors in the mature rat cochlea. Brain Res Mol Brain Res. 2000;76:25–35.
Stöver T, Nam Y, Gong TL, Lomax MI, Altschuler RA. Glial cell line-derived neurotrophic factor (GDNF) and its receptor complex are expressed in the auditory nerve of the mature rat cochlea. Hear Res. 2001;155:143–51.
Suzuki M, Yagi M, Brown JN, Miller AL, Miller JM, Raphael Y. Effect of transgenic GDNF expression on gentamicin-induced cochlear and vestibular toxicity. Gene Ther. 2000;7:1046–54.
Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, et al. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–25.
Liu Y, Okada T, Shimazaki K, Sheykholeslami K, Nomoto T, Muramatsu S, et al. Protection against aminoglycoside-induced ototoxicity by regulated AAV vector-mediated GDNF gene transfer into the cochlea. Mol Ther. 2008;16:474–80.
Scheper V, Paasche G, Miller JM, Warnecke A, Berkingali N, Lenarz T, et al. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J Neurosci Res. 2009;87:1389–99.
Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet. 1998;18:171–3.
Edery P, Attie T, Amiel J, Pelet A, Eng C, Hofstra RMW, et al. Mutation of the endothelin-3 gene in the Waardenburg-Hirschsprung disease (Shah-Waardenburg syndrome). Nat Genet. 1996;12:442–4.
Puffenberger EG, Hosoda K, Washington SS, Nakao K, deWit D, Yanagisawa M, et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell. 1994;79:1257–66.
Pardono E, van Bever Y, van den Ende J, Havrenne PC, Iughetti P, Maestrelli SRP, et al. Waardenburg syndrome: clinical differentiation between types I and II. Am J Med Genet A. 2003;117A:223–35.
Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–76.
Bagnato A, Spinella F, Rosanò L. Emerging role of the endothelin axis in ovarian tumor progression. Endocr Relat Cancer. 2005;12:761–72.
Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci USA. 1996;93:867–72.
Matsushima Y, Shinkai Y, Kobayashi Y, Sakamoto M, Kunieda T, Tachibana M. A mouse model of Waardenburg syndrome type 4 with a new spontaneous mutation of the endothelin-B receptor gene. Mamm Genome. 2002;13:30–5.
Ida-Eto M, Ohgami N, Iida M, Yajima I, Kumasaka MY, Takaiwa K, et al. Partial requirement of endothelin receptor B in spiral ganglion neurons for postnatal development of hearing. J Biol Chem. 2011;286:29621–6.
Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, et al. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–75.
Leventhall G. A review of published research on low frequency noise and its effects, Department of Environment, Food, and Rural Affairs (DEFRA), UK. 2003. http://archive.defra.gov.uk/environment/quality/noise/research/lowfrequency/documents/lowfreqnoise.pdf. Accessed 20 June 2012.
Tamura H, Ohgami N, Yajima I, Iida M, Ohgami K, Fujii N, et al. Chronic exposure to low frequency noise at moderate levels causes impaired balance in mice. PLoS ONE. 2012;7:e39807.
Al Deeb S, Al Moutaery K, Khan HA, Tariq M. Exacerbation of iminodipropionitrile-induced behavioral toxicity, oxidative stress, and vestibular hair cell degeneration by gentamicin in rats. Neurotoxicol Teratol. 2000;22:213–20.
Guthrie OW. Aminoglycoside induced ototoxicity. Toxicology. 2008;249:91–6.
Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19.
Bhattacharya A, Shukla R, Auyang ED, Dietrich KN, Bornschein R. Effect of succimer chelation therapy on postural balance and gait outcomes in children with early exposure to environmental lead. Neurotoxicology. 2007;28:686–95.
Ozcaglar HU, Agirdir B, Dinc O, Turhan M, Kilinηarslan S, Oner G. Effects of cadmium on the hearing system. Acta Otolaryngol. 2001;121:393–7.
Rice DC, Gilbert SG. Effects of developmental exposure to methyl mercury on spatial and temporal visual function in monkeys. Toxicol Appl Pharmacol. 1990;102:151–63.
Shargorodsky J, Curhan SG, Henderson E, Eavey R, Curhan GC. Heavy metals exposure and hearing loss in US adolescents. Arch Otolaryngol Head Neck Surg. 2011;137:1183–9.
Ohgami N, Kondo T, Kato M. Effects of light smoking on extra-high-frequency auditory thresholds in young adults. Toxicol Ind Health. 2011;27:143–7.
Prasher D. Heavy metals and noise exposure: health effects. Noise Health. 2009;11:141–4.
Ramirez GB, Pagulayan O, Akagi H, Francisco Rivera A, Lee LV, Berroya A, et al. Tagum study II: follow-up study at two years of age after prenatal exposure to mercury. Pediatrics. 2003;111:289–95.
Rothenberg SJ, Poblano A, Garza-Morales S. Prenatal and perinatal low level lead exposure alters brainstem auditory evoked responses in infants. Neurotoxicology. 1994;15:695–9.
Prieve BA, Yanz JL. Age-dependent changes in susceptibility to ototoxic hearing loss. Acta Otolaryngol. 1984;98:428–38.
Acknowledgments
This review is based on research that was given an encouragement award at the 82nd annual meeting of the Japanese Society for Hygiene held in Kyoto, Japan on 24–26 March 2012. We thank Yoko Kato and Harumi Ohno for their technical assistance. This study was supported in part by Grants-in-Aid for Scientific Research (B) (No. 20406003, No. 24390157 and No. 24406002) and (C) (No. 23592479), Grant-in-Aid for Challenging Exploratory Research (No. 23650241) and Grants-in-Aid for Young Scientists (B) (No. 23790160) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), AA Science Platform Program from the Japan Society for the Promotion of Science (JSPS), Adaptable and Seamless Technology Transfer Program through Target-driven R&D, Japan Science and Technology Agency, COE Project (Health Science Hills) for Private Universities from MEXT and Chubu University (No. S0801055), Research Grant from the Tokyo Biochemical Research Foundation (TBRF), the Naito Foundation Natural Science Scholarship, Research Foundation from the Institute of Science and Technology Research in Chubu University and Chubu University grants A, B and CG.
Conflict of interest
We have no financial conflict of interest in relation to this submission.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This review is based on research that was given an encouragement award at the 82nd annual meeting of the Japanese Society for Hygiene held in Kyoto, Japan on 24–26 March 2012.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ohgami, N., Iida, M., Yajima, I. et al. Hearing impairments caused by genetic and environmental factors. Environ Health Prev Med 18, 10–15 (2013). https://doi.org/10.1007/s12199-012-0300-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12199-012-0300-z