Abstract
Background
Noise, a physical factor in most work environments, has many effects on human health. Exposure to excessive noise can modify the expression of associated genes with NIHL. The aim of this study to elucidate changes in expression of GJB2 and SLC26A4 after exposure to intense noise which are the most frequent causing genes to apparent autosomal recessive non-syndromic hearing loss.
Methods
In this experimental and case–control study, 17 male Wistar rats were randomly divided into exposure groups (n = 12) and without exposure (n = 5). First group was exposed to noise (90–120 dB, 70 Hz–16 kHz, 8 h/day) for 3–6 days. Cochlear biopsies performed 1 h and 1 week post-exposure, relative gene expression levels were calculated using \({2}^{-\Delta \Delta Ct}\). From each group, one ear was stained by hematoxylin and eosin method for histopathological survey. Real-time PCR technique took place, and gene expression data were normalized by GAPDH gene. One-way ANOVA test was performed with a significance level of 0.05 by GraphPad prism software.
Results
Both GJB2 and SLC26A4 in all groups were down-regulated after exposure compared to their controls. Fold changes in the highest times were related to 1 week after 6 days of exposure, 0.052 and 0.015, respectively. Serious damages occurred in different parts of cochlea, and they were more severe after 6 days and 1-week later.
Conclusion
It is expected that if the hearing threshold tests be performed before/after exposure and considering longer post-exposure times, subsequently, the expression of these genes does not return to basal level, and irrecoverable damage to the cochlea, progressive and irreversible ARNSHL will be expected.
Similar content being viewed by others
Background
Noise, as a physical factor in most work environments, has many effects on human health, including Noise-induced hearing loss (NIHL), endocrine secretion, hypertension, stress, irritability and decreased performance [1, 2]. Over time, excessive acoustic stimulation has resulted in changes in the morphology and function of the inner ear [3]. Hearing loss (HL) is a heterogeneous disorder that has many environmental and genetic causes. Congenital hearing loss is the most common sensorineural disorder, affecting approximately one in 1000 newborns [4,5,6,7]. Previous studies have identified several genes involved in the cochlea's response to acoustic damage, which are primarily associated with various biological processes such as transcriptional control, oxidative stress, various molecular pathways and inflammation [8]. Therefore, exposure to excessive noise affects the expression of genes that seems to be important for the development of NIHL in mice [3].
To date, more than 80 various DFNB loci have been associated with autosomal recessive non-syndromic hearing loss (ARNSHL), which are considered in up to 80% of prelingual congenital deafness. Among them, DFNB1 locus harbors the most frequent cause of ARNSHL (up to 50%), the GJB2 gene (gap junction: potassium ion homeostasis, also known as CX26). DFNB4 harbors the second common gene that causes ARNSHL worldwide, SLC26A4 (anion exchanger). Regarding to this point, these genes are selected to assess the effects of noise on them [9,10,11].
Connexin26 is a member of the gap junction family, and the constituent proteins of this family are generally named based on their molecular weight. They have the highest expression in the basal and intermediate cells of the stria vascularis, the supporting cells, the spiral limbus and the spiral prominence of human and rat cochlea [5, 12,13,14]. GJB2 is a small gene that is located on 13th chromosome at position 13q12.11 in human (Homo sapiens) and on 15th chromosome at position 15p12 in rat (Rattus norvegicus). This gene consists of three exons which in both species are separated by an intron and encodes a member of the gap junction protein family [15]. CX26 is one of the key proteins involved in potassium ion homeostasis in the cochlea and is responsible for the formation of gap junctions that allow small molecules to be transferred [16]. Impairment of the CX26 gap junction complex can impair the recovery of potassium ions from synapses at the base of hair cells on the lateral wall of the cochlea [14]. Considerable changes in the endolymphatic potassium concentration have been observed in noise-induced hearing loss; therefore, CX26 could play an important role in the pathophysiological mechanisms of acoustic trauma [14].
Mutations in the SLC26A4 gene are associated with two types of ARNSHL, Pendred syndrome and DFNB4 non-syndromic hearing loss. This protein coding gene is located on 7th chromosome at position 7q22.3 in human (Homo sapiens) and on 6th chromosome at position 6q16 in rat (Rattus norvegicus) and consists of twenty-three and twenty-six exons in both species, respectively. The SLC26A4 gene encodes an anion transporter known as pendrin. The high expression of SCL26A4 in the thyroid gland, inner ear and kidney can play a role in the acid–base balance as a chloride–bicarbonate exchanger or indirectly modulate the endolymph calcium concentration [6, 17,18,19]. Pendred syndrome, a disorder usually diagnosed by Goiter, results in severe to profound HL due to a change in the inner ear at birth, and the clinical distinction between this syndrome and DFNB4 is difficult, because of the Goiter phenotype [6, 20].
To the best of our knowledge, there are only a few studies on the functional role of GJB2 (CX26) [14, 21], and no studies have been discussed on the role of SLC26A4, the genes concerned within the development of sensorineural hearing disorder in healthful animals due to exposure to environmental stimuli factor such as noise. In this study, the real-time PCR method was applied on cochlear labyrinth to elucidate the changes in the expression of GJB2 and SLC26A4 genes after exposure and also after exposure cessation to intense noise, to provide valuable insights into the design of targeted protective interventions for the prevention of non-syndromic sensorineural deafness and new clues to the pathogenesis of hearing impairment in acoustic trauma.
Methods
Study design and setting
The present study is an experimental and case–control type to investigate the association between noise exposure and changes in gene expression in male Wistar rats which was conducted in the noise laboratory of the Tehran University of Medical Sciences, School of Public Health, Department of Occupational Health Engineering, in acoustic chambers.
Characteristics of animals
Seventeen male Wistar rats at the age of 8–9 weeks, which weighed 200–250 g at study onset [22], were obtained from the animal house of Pharmacology Faculty, Tehran University of Medical Sciences, and were kept in standard noise chambers at noise and vibration laboratory of Occupational Health and Engineering Department. The ambient conditions, such as 24 h temperature (23 \(\pm \hspace{0.17em}2 ^\circ \mathrm{C}),\) humidity (50 \(\pm \hspace{0.17em}5\)%), lighting (indirectly and uniformly by the laboratory lamp), required air (air blown through three pumps) and a 12 h light/12 h dark cycle (7 a.m. to 7 p.m.), were controlled, and animals had ad libitum access to food (oval pellets) and water all the time. The rats were auditory healthy and had no problems, and then, they were randomly divided into 3 groups (2 cases and 1 control group) and were housed inside the chambers, next given a week to adjust to the new conditions.
Noise exposure
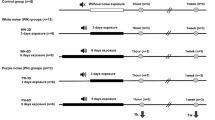
The animals were housed in 5 chambers and were allowed to move freely during exposure to noise. Control rats, without exposure (C = 5), were saved in one chamber with 50 dB background noise, and the exposed groups (Ex = 12) were sited in four chambers, three rats per each in order to reduce overlap during noise stress. The noise protocol was designed for intermittent white noise exposure for 3 and 6 days 8 h/day (Fig. 1).
The noise characteristics such as frequency band and SPL (sound pressure level) were 70 Hz–16 kHz and 90–120 dB, respectively (Fig. 1), which were produced by Cubase (5th version) and presented through a series of loudspeakers (4 per each chamber, every 20 W, 6 Ω) placed on the ceiling of the chamber and suspended 40 cm above the rats. Finally, the four speakers were routed via a headphone port outside the chamber and connected to 100 W amplifiers in order to amplify the output sound. The SPL of the noise was monitored using a sound level meter (Sound Analyzer, Model TES1358, Taipei, Taiwan).
Cochlear collection of specimens
Half of the randomly exposed rats were deeply anesthetized by the CO2 gas immediately 1 h and 1 week after exposure cessation at the end of the third day, and the remaining animals were killed at the end of the sixth day according to the above approach. Then, their head were split in two parts by Mayo scissors, brains were detached from skull and surgery was performed under a loop microscope to reach the temporal bones and cochlea. Using the external auditory meatus and the auditory nerve (paired nerve 8) as a guide, the thumb is placed inside the tympanic bulla, with an external movement and slight twist, and it is separated from other structures so that the cochlear tissue is loosened and secreted. From each group (Ex., C), the right cochlea of a rat was fixed in 4% formaldehyde for the H&E staining (overall 5 samples) and the left cochlea was frozen (overall 29 samples) for the gene expression analyses. All frozen dissections were stored at − 80 °C until processed [23].
RNA extraction procedure
Total samples (average weight of each = 50 mg) were physically disrupted by rotor–stator 5000 × g and homogenized in 250 µl of cold TRIzol lysis buffer for 90 s. The solution was incubated for 5–10 min at room temperature , and then, the amount of 250 µl chloroform was added and strongly shacked for 15 s. Microtubes were incubated on ice for 5–10 min and then centrifuged (Hettich, Germany) at 12,000 rpm for 15 min at 4 °C. The upper clear transparent aqueous phase, containing RNA, was slowly separated with a sampler and poured into another microtube and mixed with 500 µl of cold 100% isopropanol and turned the microtube inverted twice to mix well. Next, the isopropanol was completely emptied and the sediment was diluted with 250 µl of 75–80% ethanol and again centrifuged at 12,000 rpm for 5 min at 4 °C. The upper phase was then discarded and the sediment allowed to dry for 15 min at room temperature; after decolorization of the sediment, depending on its amount, 10–30 µl of DEPC or TE sterilized water was added due to the dissolution, and then, the microtubes were placed at 55 °C on dry block incubator (QIAGEN, Iran) for 15 min [23].
Quantitative RT-PCR
The buffer-mixture, RT-enzyme, primer (with a mixture of oligo (dT) and Random Hexamer) and DEPC-treated water were poured in a 0.2 microtube after centrifugation according to the instructions in the cDNA Reverse Transcription Kit (Easy™ cDNA Synthesis Kit, Parstous, Iran) per each sample and then distributed to RNA-containing microtubes. After the vortex solution, the incubation of the cDNA synthesis reaction was performed according to the temperature instructions of the corresponding kit (Table 1) in a dry block incubator (Kiagen, Iran) and finally kept at 4 °C temperature until PCR done.
To diagnose the effect of intermittent white noise on rat cochlea, the mRNA expressions of GJB2 and SLC26A4 genes in the cochlea were assessed by quantitative real-time polymerase chain reaction (qPCR). The quantitative PCR was carried out on ABI StepOne machine (USA) and StepOne software (ver. 2.0.2), using a SYBR Green kit (2X Real-Time PCR Master Mix For SYBR® Green I high ROX, BioFACT™, Korea). According to the BioFACT instruction, the 10 µl reaction was used for each well of the 48-well plate (ABI StepOne). This reaction contained 4 µl Master Mix, 4 µl DEPC-treated water, 1 µl mixed forward and reverse primers and 1 µl of cDNA sample. The primers for RT-PCR assays of GJB2 and SLC26A4 genes were extracted from the published studies [24, 25], and the GAPDH gene was designed using the Oligo7 software (Table 2). All were verified by the NCBI Primer BLAST site and then synthesized by the SinaClon Company in Iran.
The temperature conditions of the PCR were as follows [22]: one cycle with 95 °C for 10 min (denaturation I) and then 40 cycles in three steps: 95 °C for 15 s (denaturation II), 60 °C for 15 s (annealing) and 72 °C for 15 s (extension). Also, one cycle has done for melting curve in three steps: 95 °C for 15 s, 60 °C for 1 min, and then, the temperature rose by 0.3–0.3 till 95 °C. Eventually, this cycle finished at 95 °C after 15 s. The relative gene expression level was calculated using the \({2}^{-\Delta \Delta Ct}\) method and then normalized to the expression levels of the GAPDH gene. The entire process follows from The MIQE Guidelines Minimum Information for Publication of Quantitative Real-Time PCR Experiments [26].
Histopathological image analysis
The microscopic examination of a stained tissue sample on glass slides to examine the symptoms of a disease is known as histopathology [27]. Samples were prepared according to the following protocol: 1. Fixation of the samples (3cochlea) in 10% formalin for 24–72 h. 2. Dehydration by placing in 70%, 80%, 96% and 100% ethanol, respectively, 1 h each. 3. Clearing by remaining in Xylen-1 and Xylen-2, 40 min each. 4. Infiltration with wax-1 and wax-2 in the oven, 1 h each. 5. Embedding or blocking out. 6. Sectioning paraffin samples 5–10 microns thick using a machine called Microtome (Rotary Microtome, Leica Biosystems, Germany). After that, the incisions were placed in a floating tissue bath which containing alcohol solution then adhered to the lam by albumin material. 7.Paraffin removal by heating in an oven at \(95 ^\circ \mathrm{C}\) for 15 min. 8. Clearing 15 min by immersion in Xylen-1, Xylen-2 and Xylen-3. 9. Tissue hydration, 1 dip in each of 100%, 96%, 80%, 70% ethanol and washing with tap water. 10. Staining 3 min by hematoxylin then washing by tap water, staining 30 s by Eosin and washing again by tap water. 11. Dehydration by dipping 3 times in 96% and 1 dip in 100% ethanol. 12. Clearing by dipping 3 times in Xylen-1, and so on in Xylen-2 and Xylen-3. 13. Mounting the lam using Entellan™, rapid mounting medium for microscopy (Merck, Germany). 14. Imaging via light microscopy (Labomed, USA) and magnifications (4×, 10×, 40×) with the Image J software [28].
Statistical analyses
The descriptive statistics of each gene groups are presented in Tables 3 and 4. The hypothesis of normality was performed using Shapiro–Wilk statistical test, and one-way ANOVA test was used to determine the significant mean differences between groups, followed by Tukey’s test for post hoc comparisons. P value < 0.05 was considered statistical significance. All analyses were carried out with the statistical software, GraphPad Prism (Version 8, Inc., USA).
Results
Melt curves
Melting curves in all samples were sharp, single-peaks and corresponded to same temperature for all three genes, CX26, SLC26A4 and GAPDH, indicating acceptable results.
Noise exposure effects on relative gene expression of CX26 and SLC26A4
Real-time PCR assay revealed that the expression levels of GJB2 and SLC26A4 were decreased in all noise groups and post-exposure points; although this reduction in expression of both genes did not return to baseline in any of groups, the expression level of SLC26A4 increased slightly 1 week after interruption for 3 days of exposure compared to the 1 h discontinuation point, but this is not significant (Fig. 2). The expression of the SLC26A4 gene decreased significantly in 3 groups (ANOVA, F = 6.842, R square = 0.6952, P value = 0.0041), and the expression of the GJB2 gene decreased significantly 1 week after interruption for 6 days of exposure (ANOVA, F = 4.182, R square = 0.5823, P value = 0.0239) (Figs. 2, 3). Figures 2 and 3 also compare the expression levels of both genes separately in different study groups, and also their relative gene expression (fold change) is shown in Figs. 4 and 5.
Effect of intermittent white noise exposure on relative gene expression of SLC26A4 in rat cochlea. The Shapiro–Wilk normality alpha was > 0.05 for all groups. GAPDH was used for housekeeping gene. One-way ANOVA was used for statistical comparisons, and 30% of them were significant. Data were illustrated as mean ± SEM. From top of the graph *P = 0.04, *P = 0.01 and **P = 0.005
Effect of intermittent white noise exposure on relative gene expression of CX26 in rat cochlea. The Shapiro–Wilk normality alpha was > 0.05 for all groups. GAPDH was used for housekeeping gene. One-way ANOVA was used for statistical comparisons, and 10% of them were significant. Data were illustrated as mean ± SEM. *P = 0.03
Fold change of SLC26A4 gene linked to 90–120 dB SPL, 8 h/day and 70 Hz–16 kHz intermittent white noise exposure. All groups were down-regulated after exposure. Fold changes are from left to right of the graph, 0.306, 0.417, 0.122 and 0.015 compared to control, respectively, or on the other hand, the SLC26A4 gene expression in control group is 3.26, 2.4, 8.2 and 66.66 fold these groups from left to right
Fold change of CX26 gene linked to 90–120 dB SPL, 8 h/day and 70 Hz–16 kHz intermittent white noise exposure. All groups were down-regulated after exposure. Fold changes are from left to right of the graph, 0.663, 0.246, 0.147 and 0.052 compared to control, respectively, or on the other hand, the CX26 gene expression in control group is 1.5, 4.06, 6.8 and 19.23 fold these groups from left to right
Noise-induced Histological changes in the cochlea of rats that differ in their noise exposure groups
Internal/external hair cells and supporting cells in the exposed groups for 6 days showed more destruction after 1 week of cessation. In addition, damage to the basal lamina of the Reissner membrane and the auditory nerve ganglions were greater in these groups. In the exposed groups for 3 days, the rate of damage to the internal/external and supporting hair cells in 1 h after surgery was less than 1 week and this damage was less vigorous than the 6 days of exposure (Fig. 6).
Distinct histological characteristics of the rat cochlea with or without noise exposure (× 40 above, × 10 bottom). Arrows indicate different sections of cochlea. The tissues were stained by hematoxylin and eosin, and the scale bars correspond to 200 μm. A Microscopic image of control group, B surface stained image of the cochlea from Wistar-type rat, which is killed 1 h after cessation of noise exposure for 3 days versus C structure of the exposed cochlea to noise with further damage for 3 days after 1 week of discontinuation, D representative image of exposing for 6 days and killed 1 h after cessation of noise exposure versus E 6 days after noise exposure that is maintained through 7 days. Generally, damage to different sections of the cochlea against 6 day of noise was greater than in 3 days of exposure, and in both protocols, injury was more severe one week later than 1 h
As the graphs illustrated, the expression of the SLC26A4 gene at the end of the first 3 days and after 1 week of interruption with this noise did not change significantly compared to 1 h of discontinuation, but showed a very slight increase. 1 h after the end of the second 3 days, the expression of this gene decreased more than two previous groups and demonstrated the greatest deterioration a week later; therefore, both were statistically significant compared to the control group. One week after 3 days of exposure, expression of the GJB2 gene decreased more than 1 h and this down-regulation continued until one week after 6 days of exposure which expressed the only significant point compared to the normal group.
Discussion
NIHL is a complex disease influenced by personal, environmental and genetic factors. Workers exposed to similar noise levels may have different levels of hearing impairment, suggesting a genetically predisposed role [29].
Results of the study by Chung et al. in 2004 on the expression of the CX26 protein in the lateral wall of the cochlea of the male Wistar rat (continuous white noise exposure, 115 ± 5 dB, central frequency of 4 kHz for 48 consecutive hours) showed significant HL in the exposed cochlea. These parts of the findings are compatible with the present study, as the histological images showed damage to the inner and outer hair cells, Reissner membrane, supporting cells, basal lamina and damage to the auditory nerve ganglions in face of similar noise.
Another finding from Chung's study was a significant increase in CX26 protein expression in the side wall of the animal's cochlea, which is inconsistent with the results of this study. The reason for this discrepancy may be one of the following: First, in our study, the expression level of CX26 gene was measured at mRNA level by real-time PCR; however, Chung et al. tracked protein expression using Western Blot technique. Second, in the present research, the expression of CX26 gene was tested in the entire cochlea, while in the Chung’s study, the expression of this gene was calculated only in the lateral wall of the cochlea. The final cause was the animal’s lack of rest time in the Chung’s noise protocol, which was carried out continuously over 2 days (48 h); in contrast, the protocol of the present study was designed for exposure to intermittent white noise for 3–6 days (8 h/day).[14].
In 2009, Wang and colleagues investigated the effect of the CX26 protein on cochlear maturation in cCX26-transgenic null mice that lacked the GJB2 gene after birth. The main outcome of this study was the confirmation of the critical role of CX26 in cortical organ maturation after birth and prior to the onset of hearing. The results of this investigation and recent published studies indicated cell death occurs after the block of cortical organ growth and development, so CX26 plays a notable role in the maturation of the cochlea’s sensory epithelium because recycling of potassium ions by gap junctions in this area is disrupted. During acoustic stimulation, potassium ions from the endolymph penetrate the hair cells through mechanical conduction channels, and potassium ions that have accumulated around the base of the hair cells are quickly absorbed by the cochlear supporting cells, through the gap junctions and recycled back to the endolymph. The essential role of the GJB2 gene and its protein in the maturation of the Corti organ and cochlear and also its remarkable cellular role in the \({K}^{+}\) recycling pathway and exposure to noise can alter the cochlear structure and transmit the neural message, which agrees with the results of the present study [30].
Alagramam et al. conducted a study in 2014 that examined changes in gene expression using QPCR and microarray methods in the cochlea of CBA/CaJ mice that had been exposed to 110 and 116 dB noise for 1 h. Following the exposure, 243 genes were up-regulated and 61 genes were down-regulated (calcium channel genes: Cacna1b and Cacna1g). Noise exposure causes calcium to penetrate sensory cells, which promotes HL, so that down-regulation of calcium channel-dependent genes can negatively affect this mechanism and cause HL. From this finding and the discoveries of Chung’s study, it can be concluded that the reduction in the expression of the GJB2 gene and subsequent changes in the expression of the CX26 protein can influence the entrance mechanism of potassium ions into the cochlear hair cells and the conversion of mechanical signals generated by noise into neural signals and ultimately affect the recovery of these ions to the supporting cells negatively, thus promote HL [14, 31].
According to a study by Ito et al. in 2015 the reduced expression of the SLC26A4 gene and its effect on the cochlea were investigated in an animal model. The results showed degeneration and loss of stria vascular function, relatively severe and progressive HL, enlarged endolymph space and decreased endocrine cochlear potential (ECP) in 12-month-old mice, as well as thickening and edema of the striae and decreased ABR threshold in 3-month-old mice. In addition, in 12-month-old mice at the apical level of the cochlea, pathogenic heterogeneity was observed, as well as a decrease in the expression of the KCNQ1 gene, which is involved in the production of potassium channels and helps to maintain proper ionic balance for normal hearing. According to this, decreased SLC26A4 gene expression can be blamed for some damage to hair cells and spiral ganglion cells of the exposed cochlear and irreversible HL is predictable [32].
Following the previous study, the same team conducted another one on genetically modified mice in 2016, and results showed that discontinuing antibiotics prescription during the critical period led to increase HL and general degeneration of the stria vascularis occurred in one-month mice. However, re-stimulation of SLC26A4 gene expression in the cells of this segment improved the ECP and prevented auditory changes in them. Hence, the principal role of the SLC26A4 in maintaining hearing in other ototoxic conditions, such as exposure to noise in the cochlea, was shown and its reduction is considered to be the cause of hearing changes in rats [33].
In 2016, Yang et al. investigated the first genes expressed in the immune defense of Dawley rat cochleae and CBA/J mice against acoustic trauma for 2 h at 120 dB, 1–7 kHz. Among the most basic genes in the rat cochlear immune response, twelve genes from the “Solute Carrier family,” SLC25A18, SLC4A10, SLC41A3, SLC17A7, SLC7A1, SLC16A6, SLC25A29, SLC44A3, SLC16A4, SLC38A1, SLC29A23 and SLC25A25, were down-regulated, indicating a severe sensitivity of this gene family to noise exposure [8].
In 2019, Coyat et al. examined the morphological consequences of acoustic trauma in the Wistar rat cochlea, 21 days after cessation of exposure to 4–8 kHz, 119 dB single octave noise for 2 h. The histological results of Coyat’s study showed changes in the cochlea, destruction of the IHC and their fusion in the membrane of the Stereocilia, loss of spiral ganglion neurons, dislocation of the myelin sheath of the auditory nerve and permanent HL. The results of the present study and the previous one show a connection between damage to auditory nerve and internal/external hair cells and the occurrence of HL due to exposure to noise [34].
The expression of these genes in all exposure groups reduced strongly compared to their control group. Despite the significant effect in both gene groups, a remarkable influence on reducing the expression of the SLC26A4 gene occurred. It can be said that this gene is much more sensitive to noise than GJB2, so that, to understand its cause, more studies should be done on auditory, neural and even molecular pathways. Nevertheless, part of this great difference in the expression of these two genes is related to the higher expression of the SLC26A4 gene in the normal group than in the normal group of the GJB2 gene, which takes into account the standard errors 0.040077 and 0.026579, respectively.
Conclusion
Totally, down-regulated expression of both genes in all exposure groups, but one challenging group (Fig. 2) tried slightly to return to its original value after disconnection for a week. The decrease in the expression of these genes in the cochlea could be part of the endogenous mechanisms that protect the cochlea against noise exposure. Therefore, it can be expected that if the hearing threshold tests (ABR, DPOAE) be performed before and after exposure and considering the longer post-exposure times up to several months, subsequently, the expression of these genes does not return to the initial level, and serious damage to the cochlea, progressive and irreversible HL can be expected. In this study, it is assumed that the reduced gene expression is due to cochlear damage or, conversely, the cochlear damage reduced the expression of these two genes. However, it is not clear whether the decreased gene expression in the damaged area of the cochlea is temporary, or the cochlea is trying to repair the damaged areas.
The overlap of our results with previous studies examining the effects of noise exposure in other laboratories illustrated a similar trend in increasing or decreasing in the expression of various genes, and other considerable and similar issues, such as the use of animal models, (mice, chinchilla, guinea pig), type of noise (impulse, continuous, intermittent) and duration of exposure to noise or duration of interruption before tissue harvest, corroborate the method used for the present study and the consequences of noise exposure at the molecular level.
The results have a great importance in terms of occupational health because whether changes in gene expression, or histopathological images, persistent noise exposure (6 days) and its association with further destruction of hair cells, the basilar membrane of the Corti organ and other critical hearing parts in the cochlea are proved well. In addition to the changes in the expression of indispensable genes involved in the occurrence of sensorineural deafness, the likelihood of developing non-syndromic deafness in the presence of the above conditions is considered. Such changes are expected to be more pronounced in prolonged exposure to higher sound pressure levels in animals and even in humans with more symptoms.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- C:
-
Control group
- CX26/30:
-
Connexin26/30
- dB:
-
Decibel
- DFNB:
-
Deafness Neurosensory Autosomal Recessive
- Ex:
-
Exposed group
- GAPDH:
-
Glyceraldehyde 3-phosphatedehydrogenase
- GJB2:
-
Gap junction beta-2
- h:
-
Hour
- Hz:
-
Hertz
- H&E:
-
Hematoxylin and eosin
- HL:
-
Hearing loss
- IHC:
-
Internal hair cell
- kHz:
-
Kilohertz
- mRNA:
-
Messenger RNA
- NIHL:
-
Noise-induced hearing loss
- RT-PCR:
-
Real-time polymerase chain reaction
- SLC26A4:
-
Solute Carrier family 26 member A4
- Sec:
-
Second
References
Sørensen M, Wendelboe Nielsen O, Sajadieh A, Ketzel M, Tjønneland A, Overvad K et al (2017) Long-term exposure to road traffic noise and nitrogen dioxide and risk of heart failure: a cohort study. Environ Health Perspect 125(9):097021
Nassiri P, Monazzam MR, Asghari M, Zakerian SA, Dehghan SF, Folladi B et al (2014) The interactive effect of industrial noise type, level and frequency characteristics on occupational skills. Perform Enhanc Health 3(2):61–65
Gratton MA, Eleftheriadou A, Garcia J, Verduzco E, Martin GK, Lonsbury-Martin BL et al (2011) Noise-induced changes in gene expression in the cochleae of mice differing in their susceptibility to noise damage. Hearing Res 277(1–2):211–226
Falk MM (2000) Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur J Cell Biol 79(8):564–574
Lefebvre PP, VanDeWater TR (2000) Connexins, hearing and deafness: clinical aspects of mutations in the connexin 26 gene. Brain Res Rev 32(1):159–162
Shin J-W, Lee S-C, Lee H-K, Park H-J (2012) Genetic screening of GJB2 and SLC26A4 in Korean cochlear implantees: experience of soree Ear clinic. Clin Exp Otorhinolaryngol 5(Suppl 1):S10
Zelante L, Gasparini P, Estivill X, Melchionda S, D’Agruma L, Govea N et al (1997) Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet 6(9):1605–1609
Yang S, Cai Q, Vethanayagam RR, Wang J, Yang W, Hu BH (2016) Immune defense is the primary function associated with the differentially expressed genes in the cochlea following acoustic trauma. Hear Res 333:283–294
Petit C (2006) From deafness genes to hearing mechanisms: harmony and counterpoint. Trends Mol Med 12(2):57–64
Naseri M, Akbarzadehlaleh M, Masoudi M, Ahangari N, Zonouzi AAP, Zonouzi AP et al (2018) Genetic linkage analysis of DFNB4, DFNB28, DFNB93 loci in autosomal recessive non-syndromic hearing loss: evidence for digenic inheritance in GJB2 and GJB3 mutations. Iran J Public Health 47(1):95
Reiisi S, Sanati MH, Tabatabaiefar MA, Ahmadian S, Reiisi S, Parchami S et al (2014) The study of SLC26A4 gene causing autosomal recessive hearing loss by linkage analysis in a cohort of Iranian populations. Int J Mol Cell Med 3(3):176
Park HJ, Houn Hahn S, Chun YM, Park K, Kim HN (2000) Connexin26 mutations associated with nonsyndromic hearing loss. Laryngoscope 110(9):1535–1538
Van Laer L, Coucke P, Mueller R, Caethoven G, Flothmann K, Prasad S et al (2001) A common founder for the 35delG GJB2gene mutation in connexin 26 hearing impairment. J Med Genet 38(8):515–518
Hsu W-C, Wang J-D, Hsu C-J, Lee S-Y, Yeh T-H (2004) Expression of connexin 26 in the lateral wall of the rat cochlea after acoustic trauma. Acta Otolaryngol 124(4):459–463
The National Center for Biotechnology Information (NCBI) (2021) Gene category. GJB2 Gap Junction protein, Beta 2. https://www.ncbi.nlm.nih.gov/gene/2706. Accessed 9 Aug 2021, and https://www.ncbi.nlm.nih.gov/gene/394266. Accessed 6 May 2021
Wingard JC, Zhao H-B (2015) Cellular and deafness mechanisms underlying connexin mutation-induced hearing loss—a common hereditary deafness. Front Cell Neurosci 9:202
Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Adawi F et al (1997) Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17(4):411–422
Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD et al (2007) Loss of cochlear HCO3− secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol 292(5):F1345–F1353
The National Center for Biotechnology Information (NCBI) (2021) Gene category. SLC26A4 solute carrier family 26 member 4. https://www.ncbi.nlm.nih.gov/gene/5172. Accessed 19 July 2021, and https://www.ncbi.nlm.nih.gov/gene/29440. Accessed 5 June 2021
MedlinePluse (2020) Pendred syndrome. https://medlineplus.gov/genetics/condition/pendred-syndrome/. Accessed 18 Aug 2020
Zhou X-X, Chen S, Xie L, Ji Y-Z, Wu X, Wang W-W et al (2016) Reduced Connexin26 in the mature cochlea increases susceptibility to noise-induced hearing loss in mice. Int J Mol Sci 17(3):301
Bahaloo M, Rezvani ME, Yazd EF, Mehrjerdi FZ, Davari MH, Roohbakhsh A et al (2020) Effect of myricetin on the gene expressions of NOX3, TGF-β1, prestin, and HSP-70 and anti-oxidant activity in the cochlea of noise-exposed rats. Iran J Basic Med Sci 23(5):594
Rodrigues JC, de Brito Neto RV (2017) RNA extraction from Wistar Rat Cochlea for qRT-PCR. Bio Protoc. https://doi.org/10.21769/BioProtoc.2621
Tang L, Yu X, Zheng Y, Zhou N (2020) Inhibiting SLC26A4 reverses cardiac hypertrophy in H9C2 cells and in rats. PeerJ 8:e8253
Wu X, Wang Y, Sun Y, Chen S, Zhang S, Shen L et al (2014) Reduced expression of Connexin26 and its DNA promoter hypermethylation in the inner ear of mimetic aging rats induced by d-galactose. Biochem Biophys Res Commun 452(3):340–346
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M et al (2009) The MIQE Guidelines: minimum information for publication of quantitative real-time PCR experiments. Oxford University Press
Gurcan MN, Boucheron LE, Can A, Madabhushi A, Rajpoot NM, Yener B (2009) Histopathological image analysis: a review. IEEE Rev Biomed Eng 2:147–171
Feldman AT, Wolfe D (2014) Tissue processing and hematoxylin and eosin staining. In: Histopathology. Springer, pp 31–43
Zhang X, Liu Y, Zhang L, Yang Z, Shao Y, Jiang C et al (2014) Genetic variations in protocadherin 15 and their interactions with noise exposure associated with noise-induced hearing loss in Chinese population. Environ Res 135:247–252
Wang Y, Chang Q, Tang W, Sun Y, Zhou B, Li H et al (2009) Targeted connexin26 ablation arrests postnatal development of the organ of Corti. Biochem Biophys Res Commun 385(1):33–37
Alagramam KN, Stepanyan R, Jamesdaniel S, Chen DH-C, Davis RR (2014) Noise exposure immediately activates cochlear mitogen-activated protein kinase signaling. Noise Health 16(73):400
Ito T, Nishio A, Wangemann P, Griffith AJ (2015) Progressive irreversible hearing loss is caused by stria vascularis degeneration in an Slc26a4-insufficient mouse model of large vestibular aqueduct syndrome. Neuroscience 310:188–197
Nishio A, Ito T, Cheng H, Fitzgerald TS, Wangemann P, Griffith AJ (2016) Slc26a4 expression prevents fluctuation of hearing in a mouse model of large vestibular aqueduct syndrome. Neuroscience 329:74–82
Coyat C, Cazevieille C, Baudoux V, Larroze-Chicot P, Caumes B, Gonzalez-Gonzalez S (2019) Morphological consequences of acoustic trauma on cochlear hair cells and the auditory nerve. Int J Neurosci 129(6):580–587
Acknowledgements
This study is a part of the approved research project in the “Studies and Research Management Center of Tehran University of Medical Sciences” provided financial support by this center and the “Vice Presidency for Science and Technology of the Islamic Republic of Iran.”
Funding
This work was supported by “Tehran University of Medical Science” and “The Vice Presidency for Science and Technology of The Islamic Republic of Iran.”
Author information
Authors and Affiliations
Contributions
MMH contributed to data collection, statistical analysis, data interpretation and manuscript preparation, MME was supervisor and involved in design, funding and manuscript review, MKh was consultant and contributed to data linkage management, ethics, data collection, data interpretation and manuscript review, AAG was cooperator and involved in data collection and statistical analysis, and KI was supervisor and contributed to data linkage management, data collection, data interpretation and manuscript review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures involving the use and treatment of animals have been approved by the Institutional Laboratory Animal Care and Use Committee of our school and with ethics ID IR.TUMS.SPH.REC.1398.225 and thesis code 98-3-99-45875 and adhered to the ethical guidelines of the “Declaration of Helsinki”. All efforts have been made to minimize the number of animals and their suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hotkani, M.M., Esmaeilpoor, M.R.M., Khadem, M. et al. Intermittent white noise exposure is associated with rat cochleae damage and changes in the gene expression. Egypt J Med Hum Genet 23, 104 (2022). https://doi.org/10.1186/s43042-022-00317-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00317-6