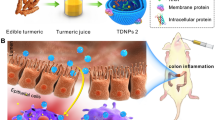
Abstract
Background
Inappropriate macrophages phenotype transition contributes to the development of ulcerative colitis, and the poly (ethylene glycol)-block-poly (d, l-lactic acid) (PEG-PLA) nanoparticles delivery system can be utilized to improve the cryptotanshinone (CTS)-based therapy.
Methods
We used a single emulsification method to prepare CTS-encapsulated nanoparticles (NPCTS). The therapeutic efficacy of NPCTS was evaluated in dextran sulfate sodium (DSS)-induced colitis mice. Then the proportion of total macrophages and M2-like macrophages were assayed with flow cytometry, and the relative content of pro-inflammatory cytokines in the colon was detected with Western blot. Bone-marrow-derived macrophages (BMDMs) were induced into M1-like macrophages, which were further incubated with NPCTS to repolarize into M2 subtype.
Results
Cryptotanshinone could induce the transition of M1 subtype to M2 subtype as indicated by up-regulated expression of arginase 1 (ARG1), interleukin (IL)-10, and CD206. In vivo, orally administrated NPCTS accumulated in the colon-infiltrated macrophages in colitis mice. It further revealed that NPCTS significantly alleviated colitis symptoms as indicated by increased body weight and colon length, decreased tumor necrosis factor (TNF)-α, IL-1β, and IL-6 content in the colon, and diminished total macrophage proportion (CD45+CD11b+F4/80+) and up-regulated M2 proportion (CD45+CD11b+F4/80+CD206hi).
Conclusion
Oral administration of NPCTS could ameliorate ulcerative colitis with the conversion of M1-like macrophages to M2-like macrophages.





Similar content being viewed by others
References
Ashrafizadeh, M., A. Zarrabi, S. Orouei, S. Saberifar, S. Salami, K. Hushmandi, and M. Najafi. Recent advances and future directions in anti-tumor activity of cryptotanshinone: a mechanistic review. Phytother. Res. 35(1):155–179, 2021
Bain, C. C., C. L. Scott, H. Uronen-Hansson, S. Gudjonsson, O. Jansson, O. Grip, M. Guilliams, B. Malissen, W. W. Agace, and A. M. Mowat. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6(3):498–510, 2013
Baumgart, D. C., and S. R. Carding. Inflammatory bowel disease: cause and immunobiology. Lancet. 369(9573):1627–1640, 2007
Chang, J. T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383(27):2652–2664, 2020
Domínguez Conde, C., and S. A. Teichmann. Deciphering immunity at high plexity and resolution. Nat. Rev. Immunol. 20(2):77–78, 2020
Don, M. J., J. F. Liao, L. Y. Lin, and W. F. Chiou. Cryptotanshinone inhibits chemotactic migration in macrophages through negative regulation of the PI3K signaling pathway. Br. J. Pharmacol. 151(5):638–646, 2007
Giacalone, G., N. Tsapis, L. Mousnier, H. Chacun, and E. Fattal. PLA-PEG nanoparticles improve the anti-inflammatory effect of rosiglitazone on macrophages by enhancing drug uptake compared to free rosiglitazone. Materials (Basel, Switzerland). 11(10):1845, 2018
Gren, S. T., and O. Grip. Role of monocytes and intestinal macrophages in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 22(8):1992–1998, 2016
He, W., N. Kapate, C. W. T. Shields, and S. Mitragotri. Drug delivery to macrophages: A review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 165:15–40, 2020
Herrlinger, K. R., and E. F. Stange. 25 years of biologicals in IBD: what’s all the hype about? J. Intern. Med. 290:806–825, 2021
Hine, A. M., and P. Loke. Intestinal macrophages in resolving inflammation. J. Immunol. 203(3):593–599, 2019
Hnatyszyn, A., S. Hryhorowicz, M. Kaczmarek-Ryś, E. Lis, R. Słomski, R. J. Scott, and A. Pławski. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered. Cancer Clin. Pract. 17:18, 2019
Kobayashi, T., B. Siegmund, C. Le Berre, S. C. Wei, M. Ferrante, B. Shen, C. N. Bernstein, S. Danese, L. Peyrin-Biroulet, and T. Hibi. Ulcerative colitis. Nat. Rev. Dis. Primers. 6(1):74, 2020
Li, H., C. Gao, C. Liu, L. Liu, J. Zhuang, J. Yang, C. Zhou, F. Feng, C. Sun, and J. Wu. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 137:111332, 2021
Liu, W., Z. Dong, K. Liu, Y. Lu, W. Wu, J. Qi, and Z. Chen. Targeting strategies of oral nano-delivery systems for treating inflammatory bowel disease. Int. J. Pharm. 600:120461, 2021
Lu, J., D. Liu, Y. Tan, R. Li, X. Wang, and F. Deng. Thalidomide attenuates colitis and is associated with the suppression of M1 macrophage polarization by targeting the transcription factor IRF5. Dig. Dis. Sci. 1:1–10, 2021
Lv, Q., Y. Xing, Y. Liu, Q. Chen, J. Xu, L. Hu, and Y. Zhang. Didymin switches M1-like toward M2-like macrophage to ameliorate ulcerative colitis via fatty acid oxidation. Pharmacol. Res. 169:105613, 2021
Moreira Lopes, T. C., D. M. Mosser, and R. Gonçalves. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm. Res. 69(12):1163–1172, 2020
Nebbia, M., N. A. Yassin, and A. Spinelli. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 33(5):305–317, 2020
De Schepper, S., S. Verheijden, J. Aguilera-Lizarraga, M. F. Viola, W. Boesmans, N. Stakenborg, I. Voytyuk, I. Schmidt, B. Boeckx, I. DierckxdeCasterle, V. Baekelandt, E. GonzalezDominguez, M. Mack, I. Depoortere, B. De Strooper, B. Sprangers, U. Himmelreich, S. Soenen, M. Guilliams, P. VandenBerghe, E. Jones, D. Lambrechts, and G. Boeckxstaens. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 175(2):400–415, 2018
Shi, M., Z. Lin, L. Ye, X. Chen, W. Zhang, Z. Zhang, F. Luo, Y. Liu, and M. Shi. Estrogen receptor-regulated SOCS3 modulation via JAK2/STAT3 pathway is involved in BPF-induced M1 polarization of macrophages. Toxicology. 433:152404, 2020
Shin, D. S., H. N. Kim, K. D. Shin, Y. J. Yoon, S. J. Kim, D. C. Han, and B. M. Kwon. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 69(1):193–202, 2009
Sung, H., J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, and F. Bray. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3):209–249, 2021
Tang, S., X. Y. Shen, H. Q. Huang, S. W. Xu, Y. Yu, C. H. Zhou, S. R. Chen, K. Le, Y. H. Wang, and P. Q. Liu. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW2647 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation. 34(2):111–118, 2011
Wang, J. L., Y. J. Gan, S. Iqbal, W. Jiang, Y. Y. Yuan, and J. Wang. Delivery of tacrolimus with cationic lipid-assisted nanoparticles for ulcerative colitis therapy. Biomater. Sci. 6(7):1916–1922, 2018
Wang, L., H. Zhang, L. Sun, W. Gao, Y. Xiong, A. Ma, X. Liu, L. Shen, Q. Li, and H. Yang. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnol. 18(1):38, 2020
Yin, Z., T. Ma, Y. Lin, X. Lu, C. Zhang, S. Chen, and Z. Jian. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell Biochem. 119(11):9419–9432, 2018
Zhang, J., Y. Zhao, T. Hou, H. Zeng, D. Kalambhe, B. Wang, X. Shen, and Y. Huang. Macrophage-based nanotherapeutic strategies in ulcerative colitis. J. Control Release. 320:363–380, 2020
Zhu, W., J. Yu, Y. Nie, X. Shi, Y. Liu, F. Li, and X. L. Zhang. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol. Invest. 43(7):638–652, 2014
Conflict of interest
Li Zhang, Longfei Yu and Yueguang Wei declare that they have no conflict of interest.
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Daqing Oilfield General Hospital. No human studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Yu, L. & Wei, Y. Oral Administration of Cryptotanshinone-Encapsulated Nanoparticles for the Amelioration of Ulcerative Colitis. Cel. Mol. Bioeng. 15, 129–136 (2022). https://doi.org/10.1007/s12195-021-00711-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-021-00711-x