Abstract
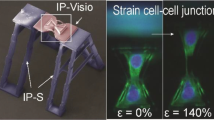
A variety of methods have been used to study the tensile properties of cells or the influence of tensile loading on cellular function. Such methods are frequently limited either by cellular detachment or by an inability to image cells at high temporal or spatial resolution. Previously, we preserved cellular adhesion during loading and imaging by using a flexible silicone membrane inverted over a glass coverslip. This enabled high magnification real-time imaging of subcellular structures but chemical and physical access to the cells was limited due to geometric constraints. In this study, we present a method to integrate thin films made from poly(dimethylsiloxane) (PDMS) into a novel device. The optically clear PDMS thin films allow simultaneous tensile loading and high magnification microscopy without the need to invert the cells, maintaining physical access during experiments. To characterize the utility of this technology, we evaluated fabrication conditions for optimizing the geometry, durability, and uniformity of these films. Additionally, we demonstrate the suitability of this device for use in high-magnification, live-cell fluorescence microscopy by examining the response of the cytoskeletal protein actin, expressed in cultured primary sensory neurons, to a tensile load. This technology offers considerable potential for extending our understanding of mechanical influences on cellular function at a variety of spatial and temporal scales.



Similar content being viewed by others
References
Armbruster, C., M. Schneider, S. Schumann, K. Gamerdinger, M. Cuevas, S. Rausch, G. Baaken, and J. Guttmann. Characteristics of highly flexible pdms membranes for long-term mechanostimulation of biological tissue. J. Biomed. Mater. Res. B 91:700–705, 2009.
Baker, B. M., R. P. Shah, A. H. Huang, and R. L. Mauck. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Eng. A 17:1445–1455, 2011.
Barbee, K. A., E. J. Macarak, and L. E. Thibault. Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation. Ann. Biomed. Eng. 22:14–22, 1994.
Bieler, F. H., C. E. Ott, M. S. Thompson, R. Seidel, S. Ahrens, D. R. Epari, U. Wilkening, K. D. Schaser, S. Mundlos, and G. N. Duda. Biaxial cell stimulation: a mechanical validation. J. Biomech. 42:1692–1696, 2009.
Bischofs, I. B., and U. S. Schwarz. Cell organization in soft media due to active mechanosensing. Proc. Natl. Acad. Sci. USA 100:9274–9279, 2003.
Bueno, F. R., and S. B. Shah. Implications of tensile loading for the tissue engineering of nerves. Tissue Eng. Part B Rev. 14:219–233, 2008.
Caille, N., Y. Tardy, and J. J. Meister. Assessment of strain field in endothelial cells subjected to uniaxial deformation of their substrate. Ann. Biomed. Eng. 26:409–416, 1998.
Camelliti, P., A. D. McCulloch, and P. Kohl. Microstructured cocultures of cardiac myocytes and fibroblasts: a two-dimensional in vitro model of cardiac tissue. Microsc. Microanal. 11:249–259, 2005.
Cesa, C. M., N. Kirchgessner, D. Mayer, U. S. Schwarz, B. Hoffmann, and R. Merkel. Micropatterned silicone elastomer substrates for high resolution analysis of cellular force patterns. Rev. Sci. Instrum. 78:034301, 2007.
Chetta, J., C. Kye, and S. B. Shah. Cytoskeletal dynamics in response to tensile loading of mammalian axons. Cytoskeleton (Hoboken) 67:650–665, 2010.
Chetta, J., and S. B. Shah. A novel algorithm to generate kymographs from dynamic axons for the quantitative analysis of axonal transport. J. Neurosci. Methods 199:230–240, 2011.
Chun, H., D. S. Lee, and H. C. Kim. Bio-cell chip fabrication and applications. Methods Mol. Biol. 509:145–158, 2009.
Davis, M. J., J. A. Donovitz, and J. D. Hood. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am. J. Physiol. 262:C1083–C1088, 1992.
Folch, A., and M. Toner. Cellular micropatterns on biocompatible materials. Biotechnol. Prog. 14:388–392, 1998.
Formigli, L., E. Meacci, C. Sassoli, R. Squecco, D. Nosi, F. Chellini, F. Naro, F. Francini, and S. Zecchi-Orlandini. Cytoskeleton/stretch-activated ion channel interaction regulates myogenic differentiation of skeletal myoblasts. J. Cell. Physiol. 211:296–306, 2007.
Garvin, J., J. Qi, M. Maloney, and A. J. Banes. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 9:967–979, 2003.
Gerstmair, A., G. Fois, S. Innerbichler, P. Dietl, and E. Felder. A device for simultaneous live cell imaging during uni-axial mechanical strain or compression. J. Appl. Physiol. 107:613–620, 2009.
Gilchrist, C. L., S. W. Witvoet-Braam, F. Guilak, and L. A. Setton. Measurement of intracellular strain on deformable substrates with texture correlation. J. Biomech. 40:786–794, 2007.
Hanein, Y., O. Tadmor, S. Anava, and A. Ayali. Neuronal soma migration is determined by neurite tension. Neuroscience 172:572–579, 2011.
Harris, A. K., P. Wild, and D. Stopak. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208:177–179, 1980.
Haston, W. S., J. M. Shields, and P. C. Wilkinson. The orientation of fibroblasts and neutrophils on elastic substrata. Exp. Cell Res. 146:117–126, 1983.
Hochmuth, R. M. Micropipette aspiration of living cells. J. Biomech. 33:15–22, 2000.
Houtchens, G. R., M. D. Foster, T. A. Desai, E. F. Morgan, and J. Y. Wong. Combined effects of microtopography and cyclic strain on vascular smooth muscle cell orientation. J. Biomech. 41:762–769, 2008.
Huang, L., P. S. Mathieu, and B. P. Helmke. A stretching device for high-resolution live-cell imaging. Ann. Biomed. Eng. 38:1728–1740, 2010.
Jean, R. P., D. S. Gray, A. A. Spector, and C. S. Chen. Characterization of the nuclear deformation caused by changes in endothelial cell shape. J. Biomech. Eng. 126:552–558, 2004.
Kartalov, E. P., W. F. Anderson, and A. Scherer. The analytical approach to polydimethylsiloxane microfluidic technology and its biological applications. J. Nanosci. Nanotechnol. 6:2265–2277, 2006.
Kaunas, R., S. Usami, and S. Chien. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell. Signal. 18:1924–1931, 2006.
Kim, B. S., and D. J. Mooney. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J. Biomech. Eng. 122:210–215, 2000.
Knight, M. M., Z. Bomzon, E. Kimmel, A. M. Sharma, D. A. Lee, and D. L. Bader. Chondrocyte deformation induces mitochondrial distortion and heterogeneous intracellular strain fields. Biomech. Model. Mechanobiol. 5:180–191, 2006.
Koschwanez, J. H., R. H. Carlson, and D. R. Meldrum. Thin PDMS films using long spin times or tert-butyl alcohol as a solvent. PLoS ONE 4:e4572, 2009.
Lawrence, C. J. The mechanics of spin coating of polymer-films. Phys. Fluids 31:2786–2795, 1988.
Lee, C. F., C. Haase, S. Deguchi, and R. Kaunas. Cyclic stretch-induced stress fiber dynamics—dependence on strain rate, rho-kinase and MLCK. Biochem. Biophys. Res. Commun. 401:344–349, 2010.
Lindqvist, N., Q. Liu, J. Zajadacz, K. Franze, and A. Reichenbach. Retinal glial (muller) cells: Sensing and responding to tissue stretch. Invest. Ophthalmol. Vis. Sci. 51:1683–1690, 2010.
Liu, M., J. R. Sun, Y. Sun, C. Bock, and Q. F. Chen. Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J. Micromech. Microeng. 19:035028, 2009.
Loverde, J. R., V. C. Ozoka, R. Aquino, L. Lin, and B. J. Pfister. Live imaging of axon stretch growth in embryonic and adult neurons. J. Neurotrauma 28:2389–2403, 2011.
Maniotis, A. J., C. S. Chen, and D. E. Ingber. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA 94:849–854, 1997.
McDonald, J. C., and G. M. Whitesides. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 35:491–499, 2002.
Merkel, R., N. Kirchgessner, C. M. Cesa, and B. Hoffmann. Cell force microscopy on elastic layers of finite thickness. Biophys. J . 93:3314–3323, 2007.
Mizutani, T., H. Haga, and K. Kawabata. Development of a device to stretch tissue-like materials and to measure their mechanical properties by scanning probe microscopy. Acta Biomater. 3:485–493, 2007.
Norrman, K., A. Ghanbari-Siahkali, and N. B. Larsen. 6 studies of spin-coated polymer films. Annu. Rep. Sect. C Phys. Chem. 101:174–201, 2005.
Paten, J. A., R. Zareian, N. Saeidi, S. A. Melotti, and J. W. Ruberti. Design and performance of an optically accessible, low-volume, mechanobioreactor for long-term study of living constructs. Tissue Eng. Part C Methods 17:775–788, 2011.
Pfister, B. J., A. Iwata, D. F. Meaney, and D. H. Smith. Extreme stretch growth of integrated axons. J. Neurosci. 24:7978–7983, 2004.
Ryu, K. S., X. Wang, K. Shaikh, and C. Liu. A method for precision patterning of silicone elastomer and its applications. J. Microelectromech. Syst. 13:568–575, 2004.
Shah, S. B., and R. L. Lieber. Simultaneous imaging and functional assessment of cytoskeletal protein connections in passively loaded single muscle cells. J. Histochem. Cytochem. 51:19–29, 2003.
Shi, Y., H. Li, X. Zhang, Y. Fu, Y. Huang, P. P. Lui, T. Tang, and K. Dai. Continuous cyclic mechanical tension inhibited runx2 expression in mesenchymal stem cells through rhoa-erk1/2 pathway. J. Cell. Physiol. 226:2159–2169, 2011.
Sia, S. K., and G. M. Whitesides. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 24:3563–3576, 2003.
Sleep, J., D. Wilson, R. Simmons, and W. Gratzer. Elasticity of the red cell membrane and its relation to hemolytic disorders: An optical tweezers study. Biophys. J. 77:3085–3095, 1999.
Smith, D. H., J. A. Wolf, T. A. Lusardi, V. M. Lee, and D. F. Meaney. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J. Neurosci. 19:4263–4269, 1999.
Smith, D. H., J. A. Wolf, and D. F. Meaney. A new strategy to produce sustained growth of central nervous system axons: continuous mechanical tension. Tissue Eng. 7:131–139, 2001.
Sniadecki, N. J., A. Anguelouch, M. T. Yang, C. M. Lamb, Z. Liu, S. B. Kirschner, Y. Liu, D. H. Reich, and C. S. Chen. Magnetic microposts as an approach to apply forces to living cells. Proc. Natl. Acad. Sci. USA 104:14553–14558, 2007.
Sonobe, T., T. Inagaki, D. C. Poole, and Y. Kano. Intracellular calcium accumulation following eccentric contractions in rat skeletal muscle in vivo: role of stretch-activated channels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294:R1329–R1337, 2008.
Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 3:413–438, 2007.
Tondon, A., H. J. Hsu, and R. Kaunas. Dependence of cyclic stretch-induced stress fiber reorientation on stretch waveform. J. Biomech. 45:728–735, 2011.
Venkataraman, S. K., L. Coyne, F. Chambon, M. Gottlieb, and H. H. Winter. Critical extent of reaction of a polydimethylsiloxane polymer network. Polymer 30:2222–2226, 1989.
Wall, M. E., P. S. Weinhold, T. Siu, T. D. Brown, and A. J. Banes. Comparison of cellular strain with applied substrate strain in vitro. J. Biomech. 40:173–181, 2007.
Wang, D., Y. Xie, B. Yuan, J. Xu, P. Gong, and X. Jiang. A stretching device for imaging real-time molecular dynamics of live cells adhering to elastic membranes on inverted microscopes during the entire process of the stretch. Integr. Biol. (Camb). 2:288–293, 2010.
Wang, J. H., and B. Li. Mechanics rules cell biology. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2:16, 2010.
Wang, Y. L., and R. J. Pelham, Jr. Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 298:489–496, 1998.
Xia, Y. N., and G. M. Whitesides. Soft lithography. Annu. Rev. Mater. Sci. 28:153–184, 1998.
Zhang, H., F. Landmann, H. Zahreddine, D. Rodriguez, M. Koch, and M. Labouesse. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471:99–103, 2011.
Zheng, J., P. Lamoureux, V. Santiago, T. Dennerll, R. E. Buxbaum, and S. R. Heidemann. Tensile regulation of axonal elongation and initiation. J. Neurosci. 11:1117–1125, 1991.
Acknowledgments
We gratefully acknowledge technical assistance by staff of the Maryland Nanocenter Micro and Nano Fabrication Laboratory and helpful discussions with the Neuromuscular Bioengineering Laboratory. This research was supported by funding from the National Science Foundation (CBET0932590 and CMMI1130997) and the State of Maryland Stem Cell Research Commission.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joyce Wong oversaw the review of this article.
B.-N. B. Nguyen and J. Chetta contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nguyen, BN.B., Chetta, J. & Shah, S.B. A Novel Technology for Simultaneous Tensile Loading and High-Resolution Imaging of Cells. Cel. Mol. Bioeng. 5, 504–513 (2012). https://doi.org/10.1007/s12195-012-0245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-012-0245-8