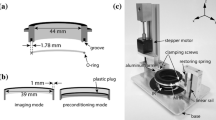
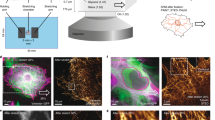
Abstract
Several custom-built and commercially available devices are available to investigate cellular responses to substrate strain. However, analysis of structural dynamics by microscopy in living cells during stretch is not readily feasible. We describe a novel stretch device optimized for high-resolution live-cell imaging. The unit assembles onto standard inverted microscopes and applies constant magnitude or cyclic stretch at physiological magnitudes to cultured cells on elastic membranes. Interchangeable modular indenters enable delivery of equibiaxial and uniaxial stretch profiles. Strain analysis performed by tracking fluorescent microspheres adhered onto the substrate demonstrated reproducible application of stretch profiles. In endothelial cells transiently expressing enhanced green fluorescent protein (EGFP)-vimentin and paxillin-DsRed2 and subjected to constant magnitude equibiaxial stretch, the two-dimensional strain tensor demonstrated efficient transmission through the extracellular matrix and focal adhesions. Decreased transmission to the intermediate filament network was measured, and a heterogeneous spatial distribution of maximum stretch magnitude revealed discrete sites of strain focusing. Spatial correlation of vimentin and paxillin displacement vectors provided an estimate of the extent of mechanical coupling between the structures. Interestingly, switching the spatial profile of substrate strain reveals that actin-mediated edge ruffling is not desensitized to repeated mechanostimulation. These initial observations show that the stretch device is compatible with live-cell microscopy and is a novel tool for measuring dynamic structural remodeling under mechanical strain.









Similar content being viewed by others
Abbreviations
- EGFP:
-
Enhanced green fluorescent protein
- FA:
-
Focal adhesion
- IF:
-
Intermediate filament
- IR:
-
Indenter ring
- MHR:
-
Membrane holder ring
- MP:
-
Mobile plate
References
Arold, S. P., J. Y. Wong, and B. Suki. Design of a new stretching apparatus and the effects of cyclic strain and substratum on mouse lung epithelial-12 cells. Ann. Biomed. Eng. 35:1156–1164, 2007.
Barbee, K. A., E. J. Macarak, and L. E. Thibault. Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation. Ann. Biomed. Eng. 22:14–22, 1994.
Brown, C. M., B. Hebert, D. L. Kolin, J. Zareno, L. Whitmore, A. R. Horwitz, and P. W. Wiseman. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell Sci. 119:5204–5214, 2006.
Caille, N., Y. Tardy, and J. J. Meister. Assessment of strain field in endothelial cells subjected to uniaxial deformation of their substrate. Ann. Biomed. Eng. 26:409–416, 1998.
Choi, C. K., and B. P. Helmke. Short-term shear stress induces rapid actin dynamics in living endothelial cells. Mol. Cell. Biomech. 5:247–258, 2008.
Clark, C. B., T. J. Burkholder, and J. A. Frangos. Uniaxial strain system to investigate strain rate regulation in vitro. Rev. Sci. Instrum. 72:2415–2422, 2001.
Geddes, D. M., and R. S. Cargill, 2nd. An in vitro model of neural trauma: Device characterization and calcium response to mechanical stretch. J. Biomech. Eng. 123:247–255, 2001.
Haga, J. H., Y. S. Li, and S. Chien. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J. Biomech. 40:947–960, 2007.
Helmke, B. P. Molecular control of cytoskeletal mechanics by hemodynamic forces. Physiology 20:43–53, 2005.
Helmke, B. P., R. D. Goldman, and P. F. Davies. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ. Res. 86:745–752, 2000.
Helmke, B. P., A. B. Rosen, and P. F. Davies. Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys. J. 84:2691–2699, 2003.
Hiraoka, Y., J. W. Sedat, and D. A. Agard. Determination of three-dimensional properties of a light microscope system: Partial confocal behavior in epifluorescence microscopy. Biophys. J. 57:325–333, 1990.
Hu, K., L. Ji, K. T. Applegate, G. Danuser, and C. M. Waterman-Storer. Differential transmission of actin motion within focal adhesions. Science 315:111–115, 2007.
Hung, C. T., and J. L. Williams. A method for inducing equi-biaxial and uniform strains in elastomeric membranes used as cell substrates. J. Biomech. 27:227–232, 1994.
Iwayoshi, S., K. Furukawa, and T. Ushida. Continuous visualization of morphological changes in endothelial cells in response to cyclic stretch. JSME Int. J. Ser. C, Mech. Syst. Mach. Elem. Manuf. 49:545–555, 2006.
Katsumi, A., J. Milanini, W. B. Kiosses, M. A. Del Pozo, R. Kaunas, S. Chien, K. M. Hahn, and M. A. Schwartz. Effects of cell tension on the small GTPase Rac. J. Cell Biol. 158:153–164, 2002.
Keller, R., D. Shook, and P. Skoglund. The forces that shape embryos: Physical aspects of convergent extension by cell intercalation. Phys. Biol. 5:15007, 2008.
Lee, A. A., T. Delhaas, L. K. Waldman, D. A. Mackenna, F. J. Villarreal, and A. D. Mcculloch. An equibiaxial strain system for cultured cells. Am. J. Physiol. 271:C1400–C1408, 1996.
Li, Y. S., J. H. Haga, and S. Chien. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 38:1949–1971, 2005.
Moretti, M., A. Prina-Mello, A. J. Reid, V. Barron, and P. J. Prendergast. Endothelial cell alignment on cyclically-stretched silicone surfaces. J. Mater. Sci.: Mater. Med. 15:1159–1164, 2004.
Mott, R. E., and B. P. Helmke. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am. J. Physiol. 293:C1616–C1626, 2007.
Orr, A. W., B. P. Helmke, B. R. Blackman, and M. A. Schwartz. Mechanisms of mechanotransduction. Dev. Cell 10:11–20, 2006.
Poh, Y.-C., S. Na, F. Chowdhury, M. Ouyang, Y. Wang, and N. Wang. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS ONE 4:e7886, 2009.
Rana, O. R., C. Zobel, E. Saygili, K. Brixius, F. Gramley, T. Schimpf, K. Mischke, D. Frechen, C. Knackstedt, R. H. Schwinger, and P. Schauerte. A simple device to apply equibiaxial strain to cells cultured on flexible membranes. Am. J. Physiol. 294:H532–H540, 2008.
Schaffer, J. L., M. Rizen, G. J. L’italien, A. Benbrahim, J. Megerman, L. C. Gerstenfeld, and M. L. Gray. Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell culture membrane. J. Orthop. Res. 12:709–719, 1994.
Schwartz, M. A., and D. W. Desimone. Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 20:551–556, 2008.
Sotoudeh, M., S. Jalali, S. Usami, J. Y. J. Shyy, and S. Chien. A strain device imposing dynamic and uniform equi-biaxial strain to cultured cells. Ann. Biomed. Eng. 26:181–189, 1998.
Waters, C. M., M. R. Glucksberg, E. P. Lautenschlager, C.-W. Lee, R. M. Van Matre, R. J. Warp, U. Savla, K. E. Healy, B. Moran, D. G. Castner, and J. P. Bearinger. A system to impose prescribed homogenous strains on cultured cells. J. Appl. Physiol. 91:1600–1610, 2001.
Acknowledgments
The authors thank Drs. Martin Schwartz and Doug DeSimone for helpful discussions during the design of the stretch device. This study was supported by a grant from the UVa Office of the Vice President for Research and Graduate Studies and by NIH grants HL-071958 and HL-080956.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Scott I. Simon oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Huang, L., Mathieu, P.S. & Helmke, B.P. A Stretching Device for High-Resolution Live-Cell Imaging. Ann Biomed Eng 38, 1728–1740 (2010). https://doi.org/10.1007/s10439-010-9968-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9968-7