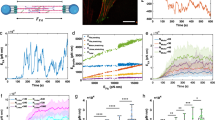
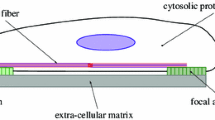
Abstract
Cyclic stretching of adherent cells regulates cell morphology, signal transduction and cell function. It is well established that actin stress fibers are mechano-sensitive structural elements that reorganize in response to applied stress and strain. In this review, we discuss studies revealing the roles of myosin II in stress fiber remodeling including stress fiber assembly, disassembly, and tension maintenance. The results of these studies are interpreted with mathematical models that describe a mechanism by which stress fibers reorganize in response to cyclic stretch and predict how changes in stress fiber tension regulate signal transduction dependent on the spatial and temporal patterns of the applied strain.






Similar content being viewed by others
References
Almeida, E. A., D. Ilic, Q. Han, C. R. Hauck, F. Jin, H. Kawakatsu, D. D. Schlaepfer, and C. H. Damsky. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J. Cell Biol. 149:741–754, 2000.
Balaban, N. Q., U. S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472, 2001.
Bershadsky, A. D., N. Q. Balaban, and B. Geiger. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19:677–695, 2003.
Brown, R. A., R. Prajapati, D. A. McGrouther, I. V. Yannas, and M. Eastwood. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 175:323–332, 1998.
Chien, S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 292:H1209–H1224, 2007.
Chrzanowska-Wodnicka, M., and K. Burridge. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133:1403–1415, 1996.
Civelekoglu, G., Y. Tardy, and J. J. Meister. Modeling actin filament reorganization in endothelial cells subjected to cyclic stretch. Bull. Math. Biol. 60:1017–1037, 1998.
Colombelli, J., A. Besser, H. Kress, E. G. Reynaud, P. Girard, E. Caussinus, U. Haselmann, J. V. Small, U. S. Schwarz, and E. H. Stelzer. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell Sci. 122:1665–1679, 2009.
Costa, K. D., W. J. Hucker, and F. C. Yin. Buckling of actin stress fibers: a new wrinkle in the cytoskeletal tapestry. Cell Motil. Cytoskeleton 52:266–274, 2002.
Cramer, L. P., M. Siebert, and T. J. Mitchison. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J. Cell Biol. 136:1287–1305, 1997.
De, R., A. Zemel, and S. A. Safran. Dynamics of cell orientation. Nat. Phys. 3:655–659, 2007.
Deguchi, S., T. Ohashi, and M. Sato. Evaluation of tension in actin bundle of endothelial cells based on preexisting strain and tensile properties measurements. Mol. Cell Biomech. 2:125–133, 2005.
DeMali, K. A., K. Wennerberg, and K. Burridge. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15:572–582, 2003.
Gavara, N., P. Roca-Cusachs, R. Sunyer, R. Farre, and D. Navajas. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. Biophys. J. 95:464–471, 2008.
Goldyn, A. M., B. A. Rioja, J. P. Spatz, C. Ballestrem, and R. Kemkemer. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J. Cell Sci. 122:3644–3651, 2009.
Hahn, C., and M. A. Schwartz. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 10:53–62, 2009.
Haviv, L., D. Gillo, F. Backouche, and A. Bernheim-Groswasser. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J. Mol. Biol. 375:325–330, 2008.
Hayakawa, K., N. Sato, and T. Obinata. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp. Cell Res. 268:104–114, 2001.
Hayakawa, K., H. Tatsumi, and M. Sokabe. Loss of mechanical tension causes ADF/cofilin mediated stress fiber disassembly in cultured endothelial cells: a study by use of a semi-intact cell system. Cell Struct. Funct. 29:S19, 2004.
He, Y., and F. Grinnell. Stress relaxation of fibroblasts activates a cyclic AMP signaling pathway. J. Cell Biol. 126:457–464, 1994.
Hill, A. V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B Biol. Sci. 126:136–195, 1938.
Hirata, H., H. Tatsumi, and M. Sokabe. Dynamics of actin filaments during tension-dependent formation of actin bundles. Biochim. Biophys. Acta 1115–1127:2007, 1770.
Hirata, H., H. Tatsumi, and M. Sokabe. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J. Cell Sci. 121:2795–2804, 2008.
Hornberger, T. A., D. D. Armstrong, T. J. Koh, T. J. Burkholder, and K. A. Esser. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. Am. J. Physiol. Cell Physiol. 288:C185–C194, 2005.
Hotulainen, P., and P. Lappalainen. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173:383–394, 2006.
Hotulainen, P., E. Paunola, M. K. Vartiainen, and P. Lappalainen. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol. Biol. Cell 16:649–664, 2005.
Hsu, H. J., C. F. Lee, and R. Kaunas. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One 4:e4853, 2009.
Hsu, H. J., C. F. Lee, A. Locke, S. Q. Vanderzyl, and R. Kaunas. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One 5:e12470, 2010.
Jungbauer, S., H. Gao, J. P. Spatz, and R. Kemkemer. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J. 95:3470–3478, 2008.
Katoh, K., Y. Kano, M. Amano, K. Kaibuchi, and K. Fujiwara. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am. J. Physiol. Cell Physiol. 280:C1669–C1679, 2001.
Katsumi, A., T. Naoe, T. Matsushita, K. Kaibuchi, and M. A. Schwartz. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J. Biol. Chem. 280:16546–16549, 2005.
Kaunas, R., H. J. Hsu, and S. Deguchi. Sarcomeric model of stretch-induced stress fiber reorganization. Cell Health Cytoskeleton 3:13–22, 2011.
Kaunas, R., P. Nguyen, S. Usami, and S. Chien. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl Acad. Sci. USA 102:15895–15900, 2005.
Kaunas, R., S. Usami, and S. Chien. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell. Signal. 18:1924–1931, 2006.
Kawakami, K., H. Tatsumi, and M. Sokabe. Dynamics of integrin clustering at focal contacts of endothelial cells studied by multimode imaging microscopy. J. Cell Sci. 114:3125–3135, 2001.
Kumar, S., I. Z. Maxwell, A. Heisterkamp, T. R. Polte, T. P. Lele, M. Salanga, E. Mazur, and D. E. Ingber. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90:3762–3773, 2006.
Kushida, N., Y. Kabuyama, O. Yamaguchi, and Y. Homma. Essential role for extracellular Ca(2+) in JNK activation by mechanical stretch in bladder smooth muscle cells. Am. J. Physiol. Cell Physiol. 281:C1165–C1172, 2001.
Langanger, G., M. Moeremans, G. Daneels, A. Sobieszek, M. De Brabander, and J. De Mey. The molecular organization of myosin in stress fibers of cultured cells. J. Cell Biol. 102:200–209, 1986.
Lazarides, E., and K. Burridge. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell 6:289–298, 1975.
Lee, C. F., C. Haase, S. Deguchi, and R. Kaunas. Cyclic stretch-induced stress fiber dynamics—dependence on strain rate, Rho-kinase and MLCK. Biochem. Biophys. Res. Commun. 401:344–349, 2010.
Lu, L., Y. Feng, W. J. Hucker, S. J. Oswald, G. D. Longmore, and F. C. Yin. Actin stress fiber pre-extension in human aortic endothelial cells. Cell Motil. Cytoskeleton 65:281–294, 2008.
Maciver, S. K., H. G. Zot, and T. D. Pollard. Characterization of actin filament severing by actophorin from Acanthamoeba castellanii. J. Cell Biol. 115:1611–1620, 1991.
MacKenna, D. A., F. Dolfi, K. Vuori, and E. Ruoslahti. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J. Clin. Invest. 101:301–310, 1998.
Matsui, T. S., K. Ito, R. Kaunas, M. Sato, and S. Deguchi. Actin stress fibers are at a tipping point between conventional shortening and rapid disassembly at physiological levels of MgATP. Biochem. Biophys. Res. Commun. 395:301–306, 2010.
Mizutani, T., H. Haga, and K. Kawabata. Cellular stiffness response to external deformation: tensional homeostasis in a single fibroblast. Cell Motil. Cytoskeleton 59:242–248, 2004.
Neidlinger-Wilke, C., E. S. Grood, J.-C. Wang, R. A. Brand, and L. Claes. Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. J. Orthop. Res. 19:286–293, 2001.
Park, J. S., J. S. Chu, C. Cheng, F. Chen, D. Chen, and S. Li. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol. Bioeng. 88:359–368, 2004.
Polte, T. R., G. S. Eichler, N. Wang, and D. E. Ingber. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 286:C518–C528, 2004.
Ridley, A. J., and A. Hall. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399, 1992.
Riveline, D., E. Zamir, N. Q. Balaban, U. S. Schwarz, T. Ishizaki, S. Narumiya, Z. Kam, B. Geiger, and A. D. Bershadsky. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153:1175–1186, 2001.
Russell, R. J., S. L. Xia, R. B. Dickinson, and T. P. Lele. Sarcomere mechanics in capillary endothelial cells. Biophys. J. 97:1578–1585, 2009.
Sanger, J. W., J. M. Sanger, and B. M. Jockusch. Differences in the stress fibers between fibroblasts and epithelial cells. J. Cell Biol. 96:961–969, 1983.
Sato, K., T. Adachi, M. Matsuo, and Y. Tomita. Quantitative evaluation of threshold fiber strain that induces reorganization of cytoskeletal actin fiber structure in osteoblastic cells. J. Biomech. 38:1895–1901, 2005.
Sato, K., T. Adachi, Y. Shirai, and N. Saito. Local disassembly of actin stress fibers induced by selected release of intracellular tension in osteoblastic cells. J. Biomech. Sci. Eng. 1:204–214, 2006.
Sawada, Y., and M. P. Sheetz. Force transduction by Triton cytoskeletons. J. Cell Biol. 156:609–615, 2002.
Sawada, Y., M. Tamada, B. J. Dubin-Thaler, O. Cherniavskaya, R. Sakai, S. Tanaka, and M. P. Sheetz. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127:1015–1026, 2006.
Schoenwaelder, S. M., and K. Burridge. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 11:274–286, 1999.
Simpson, D. G., M. Majeski, T. K. Borg, and L. Terracio. Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ. Res. 85:e59–e69, 1999.
Smith, M. A., E. Blankman, M. L. Gardel, L. Luettjohann, C. M. Waterman, and M. C. Beckerle. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev. Cell 19:365–376, 2010.
Smith, P. G., C. Roy, Y. N. Zhang, and S. Chauduri. Mechanical stress increases RhoA activation in airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 28:436–442, 2003.
Stachowiak, M. R., and B. O’Shaughnessy. Recoil after severing reveals stress fiber contraction mechanisms. Biophys. J. 97:462–471, 2009.
Stamenovic, D., K. A. Lazopoulos, A. Pirentis, and B. Suki. Mechanical stability determines stress fiber and focal adhesion orientation. Cell. Mol. Bioeng. 2:475–485, 2009.
Takei, T., C. Rivas-Gotz, C. A. Delling, J. T. Koo, I. Mills, T. L. McCarthy, M. Centrella, and B. E. Sumpio. Effect of strain on human keratinocytes in vitro. J. Cell. Physiol. 173:64–72, 1997.
Takemasa, T., T. Yamaguchi, Y. Yamamoto, K. Sugimoto, and K. Yamashita. Oblique alignment of stress fibers in cells reduces the mechanical stress in cyclically deforming fields. Eur. J. Cell Biol. 77:91–99, 1998.
Tanner, K., A. Boudreau, M. J. Bissell, and S. Kumar. Dissecting regional variations in stress fiber mechanics in living cells with laser nanosurgery. Biophys. J. 99:2775–2783, 2010.
Totsukawa, G., Y. Yamakita, S. Yamashiro, D. J. Hartshorne, Y. Sasaki, and F. Matsumura. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 150:797–806, 2000.
Wang, J. H. Substrate deformation determines actin cytoskeleton reorganization: a mathematical modeling and experimental study. J. Theor. Biol. 202:33–41, 2000.
Watanabe, N., T. Kato, A. Fujita, T. Ishizaki, and S. Narumiya. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1:136–143, 1999.
Wei, Z., V. S. Deshpande, R. M. McMeeking, and A. G. Evans. Analysis and interpretation of stress fiber organization in cells subject to cyclic stretch. J. Biomech. Eng. 130:031009, 2008.
Wilson, C. A., M. A. Tsuchida, G. M. Allen, E. L. Barnhart, K. T. Applegate, P. T. Yam, L. Ji, K. Keren, G. Danuser, and J. A. Theriot. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature 465:373–377, 2010.
Yin, H. L., and T. P. Stossel. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature 281:583–586, 1979.
Acknowledgments
The authors were supported by grants from the American Heart Association (0730238N) and the National Science Foundation (CBET-0854129) to RK and by Special Coordination Funds for Promoting Science and Technology and Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#21680039) to SD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Yingxiao Wang & Peter J. Butler oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Kaunas, R., Deguchi, S. Multiple Roles for Myosin II in Tensional Homeostasis Under Mechanical Loading. Cel. Mol. Bioeng. 4, 182–191 (2011). https://doi.org/10.1007/s12195-011-0175-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-011-0175-x