Abstract
It is well documented in a variety of adherent cell types that in response to anisotropic signals from the microenvironment cells alter their cytoskeletal organization. Previous theoretical studies of these phenomena were focused primarily on the elasticity of cytoskeletal actin stress fibers (SFs) and of the substrate while the contribution of focal adhesions (FAs) through which the cytoskeleton is linked to the external environment has not been considered. Here we propose a mathematical model comprised of a single linearly elastic SF and two identical linearly elastic FAs of a finite size at the endpoints of the SF to investigate cytoskeletal realignment in response to uniaxial stretching of the substrate. The model also includes the contribution of the chemical potential energies of the SF and the FAs to the total potential energy of the SF–FA assembly. Using the global (Maxwell’s) stability criterion, we predict stable configurations of the SF–FA assembly in response to substrate stretching. Model predictions obtained for physiologically feasible values of model parameters are consistent with experimental data from the literature. The model shows that elasticity of SFs alone can not predict their realignment during substrate stretching and that geometrical and elastic properties of SFs and FAs need to be included.







Similar content being viewed by others
Notes
Pure uniaxial stretching corresponds to the case where cells are deformed in the direction of substrate stretching and not in the perpendicular direction, as opposed to the simple uniaxial stretching where the cells are deformed in both the direction of stretching and in the perpendicular direction due to the Poisson’s effect.
Abbreviations
- SF:
-
Stress fiber
- FA:
-
Focal adhesion
- CSK:
-
Cytoskeleton
References
Balaban, N. Q., U. S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472, 2001.
Bausch, A. R., F. Ziemann, A. A. Boulbitch, K. Jacobson, and E. Sackmann. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead rheometry. Biophys. J. 75:2038–2049, 1998.
Costa, K. D., W. J. Hucker, and F. C.-P. Yin. Buckling of actin stress fibers: a new wrinkle in the cytoskeletal tapestry. Cell Motil. Cytoskeleton 52:266–274, 2002.
Dartsch, P. C., and H. Hämmerle. Orientation response of arterial smooth muscle cells to mechanical stimulation. Eur. J. Cell Biol. 41:339–346, 1986.
De, R., A. Zemel, and S. A. Safran. Dynamics of cell orientation. Nat. Phys. 3:655–659, 2007.
Deguchi, S., S. Ohashi, and M. Sato. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech. 39:2603–2610, 2006.
Ericksen, J. L. Introduction to the Thermodynamics of Solids, Chapter 3, edited by R. J. Knops, and K. W. Morton. London: Chapman & Hall. pp. 39–61.
Franz, C. M., and D. J. Müller. Analyzing focal adhesion structure by atomic force microscopy. J. Cell Sci. 118:5315–5323, 2005.
Friedl, P., and E.-B. Bröcker. The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci. 57:41–64, 2000.
Grinnell, F., C.-H. Ho, E. Tamariz, D. J. Lee, and G. Skuta. Dendritic fibroblasts in three-dimensional collagen matrix. Mol. Biol. Cell 14:384–395, 2003.
Hayakawa, K., N. Sato, and T. Obinata. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp. Cell Res. 268:104–114, 2001.
Hill, T. L. Microfilament or microtubule assembly or disassembly against a force. Proc. Natl Acad. Sci. USA 78:5613–5617, 1981.
Hsu, H.-J., C.-F. Lee, and R. Kaunas. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One 4:e4853, 2009.
Iba, T., and B. E. Sumpio. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc. Res. 42:245–254, 1991.
Kaunas, R., P. Nguyen, S. Usami, and S. Chien. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl Acad. Sci. USA 102:15895–15900, 2005.
Kaverina, I., O. Krylyshkina, K. Beningo, K. Anderson, Y.-L. Wang, and J. V. Small. Tensile stress stimulates microtubule outgrowth in living cells. J. Cell Sci. 115:2283–2291, 2002.
Kumar, S., I. Z. Maxwell, A. Heisterkamp, T. R. Polte, T. P. Lele, M. Salanga, E. Mazur, and D. E. Ingber. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90:3762–3773, 2006.
Kurpinski, K., J. Chu, C. Hashi, and S. Li. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl Acad. Sci. USA 103:16095–16100, 2006.
Lazopoulos, K. A., and A. Pirentis. Substrate stretching and reorganization of stress fibers as a finite elasticity problem. Int. J. Solids Struct. 44:8285–8296, 2007.
Lazopoulos, K. A., and D. Stamenović. Durotaxis as an elastic stability phenomenon. J. Biomech. 41:1289–1294, 2008.
Lu, L., Y. Feng, W. J. Hucker, S. J. Oswald, G. D. Longmore, and F. C.-P. Yin. Actin stress fiber pre-extension in human aortic endothelial cells. Cell Motil. Cytoskeleton 65:281–294, 2008.
Neidlinger-Wilke, C., E. S. Grood, J. H.-C. Wang, R. A. Brand, and L. Claes. Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. J. Orthop. Res. 19:286–293, 2001.
Overby, D. R., B. D. Matthews, E. Alsberg, and D. E. Ingber. Novel dynamic rheological behavior of individual focal adhesions measured within single cells using electromagnetic pulling cytometry. Acta Biomater. 1:295–303, 2005.
Shemesh, T., B. Geiger, A. D. Bershadsky, and M. M. Kozlov. Focal adhesions as mechanosensors: a physical mechanism. Proc. Natl Acad. Sci. USA 102:12383–12388, 2005.
Sipkema, P., P. J. W. van der Linden, N. Westerhof, and F. C.-P. Yin. Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J. Biomech. 36:653–659, 2003.
Smith, P. G., R. Garcia, and L. Kogerman. Mechanical strain increases protein tyrosine phosphorylation in airway smooth muscle cells. Exp. Cell Res. 239:353–360, 1998.
Stamenović, D., and D. E. Ingber. Tensegrity-guided self assembly: from molecules to living cells. Soft Matter 5:1137–1145, 2009.
Takemasa, T., K. Sugimoto, and K. Yamashita. Amplitude-dependent stress fiber reorientation in early response to cyclic strain. Exp. Cell Res. 230:407–410, 1997.
Wang, J. H.-C. Substrate deformation determines actin cytoskeleton reorganization: mathematical modeling and experimental study. J. Theor. Biol. 202:33–41, 2000.
Wang, J. H.-C., P. Goldschmidt-Clermont, J. Wille, and F. C.-P. Yin. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 34:1563–1572, 2001.
Wang, J. H.-C., P. Goldschmidt-Clermont, and F. C.-P. Yin. Contractility affects stress fiber remodeling and reorientation of endothelial cells subjected to cyclic mechanical stretching. Ann. Biomed. Eng. 28:1165–1171, 2000.
Wei, Z., V. S. Deshpande, R. M. McMeeking, and A. G. Evans. Analysis and interpretation of stress fiber organization in cells subjected to cyclic stretching. ASME J. Biomech. Eng. 130:031009, 2008.
Wille, J. J., C. A. Ambrosi, and F. C.-P. Yin. Comparison of the effects of cyclic stretching and compression on endothelial cell morphological responses. ASME J. Biomech. Eng. 126:545–551, 2004.
Yoshigi, M., L. M. Hoffman, C. C. Jensen, H. J. Yost, and M. C. Beckerle. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 171:209–215, 2005.
Acknowledgments
We thank H. Parameswaran for his technical help and A. Majumdar for helpful discussions. This work is supported by the Coulter Foundation grant and by the National Heart, Blood and Lung Institute Grant HL 096005.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Derivation of Eq. (6)
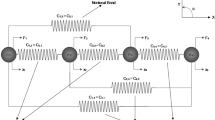
We assume a one-dimensional state of stress in both the SF and FAs in the direction of the SF axis. We further assume, based on the one-dimensional model of FA by Shemesh et al. 24 that at the distal end (i.e., ξ = 0) the FA resist the traction T, and at the proximal end the FA resists the pulling stress σ of the SF. Assuming that the pulling stress from the SF and the traction from the substrate are transmitted to the FA linearly along its length L FA, it follows that at a distance ξ from the distal end the stress transmitted from the SF to the FA is σξ = (ξ/L FA)σ and the traction transmitted from the substrate to the FA is T ξ = (1 − ξ/L FA)T (see Fig. 1). While both the traction and the SF stress are transmitted to the FA over L FA, the corresponding contact SF–FA and FA-substrate areas are not the same. This is necessary to provide consistency of the force balance of the free-body diagram shown in Fig. 1.
Mechanical equilibrium demands that at every point ξ of the FA the stresses τξ, σξ, and T ξ are balanced, i.e., that σξ = τξ + T ξ, from which we obtain that τξ = σξ − T ξ = (σ + T)(ξ/L FA) − T. Using this expression, we obtain the average stress τ within the FA as follows
Equilibrium of the whole SF–FA assembly requires that the tensile force due to σ and the force due to the traction are balanced, i.e., that
where A SF is the cross-sectional area of the SF, A FA is the interfacial area of the FA, and b is a constant, representing the width of the FA at the interface with the substrate, i.e., A FA = bL FA. By substituting the expression for T ξ into Eq. (A2), we obtain that
By substituting Eq. (A3) into Eq. (A1), we obtain Eq. (6).
Derivation of Eq. (10)
To obtain a relationship between u n , u 0, and u x for simple uniaxial stretching, we follow the steps described in Lazopoulos and Pirentis.19 We assume that the SF is in the substrate xy-plane, at angle θ with respect to the x-axis, and that it carries an initial pre-strain u 0. The substrate is stretched uniaxially in the direction of the x-axis, with the displacement gradient u x (Fig. 2). Due to the Poisson’s effect, the substrate also deforms in the y-direction with the displacement gradient u y = −νu x , where ν is the Poisson’s ratio. The deformation gradient tensor F of the SF is a product of the deformation gradient of the substrate F sub and the deformation gradient F 0 of the SF due to the pre-strain, where xy-Cartesian components of F sub and F 0 are given as follows
From Eqs. (A4), we obtain F = F sub F 0. Using the right Cauchy-Green strain tensor C = F T F, where the superscript T indicates transpose of tensor, we calculate the displacement gradient (u n ) of the SF as follows \( u_{n} = \sqrt {{\mathbf{Cn}} \cdot {\mathbf{n}}} - 1, \) where n = (cos θ, sin θ) is a unit vector parallel with the SF (see Fig. 2), as follows
For small u x , i.e., u x ≪ 1 and u x u 0 ≪ 1, Eq. (A5) can be linearized and we obtain u n as given by Eq. (10).
Rights and permissions
About this article
Cite this article
Stamenović, D., Lazopoulos, K.A., Pirentis, A. et al. Mechanical Stability Determines Stress Fiber and Focal Adhesion Orientation. Cel. Mol. Bioeng. 2, 475–485 (2009). https://doi.org/10.1007/s12195-009-0093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-009-0093-3