Abstract
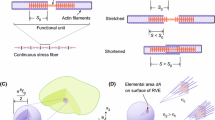
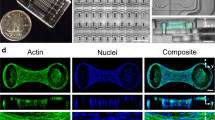
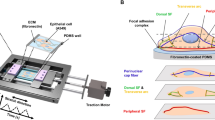
Stress fibers in the cytoskeleton are essential in maintaining cellular shape and influence cellular adhesion and migration. Cyclic uniaxial stretching results in cellular reorientation orthogonal to the applied stretch direction. The mechanistic cues underlying changes to cellular form and function to stretch stimuli are currently underexplored. We show stretch-induced stress fiber lengthening, their realignment, and increased cortical actin in NIH 3T3 fibroblasts stretched over varied amplitudes and durations. Higher amounts of actin and stress fiber alignment were accompanied with an increase in the effective elastic modulus of cells. Microtubules did not contribute to the measured stiffness or reorientation response but were essential to the nuclear reorientation. We used a phenomenological growth and remodeling law, based on the experimental data, to model stress fiber elongation and reorientation dynamics based on a nonlinear, orthotropic, fiber-reinforced continuum representation of the cell. The model predicts the changes observed fibroblast morphology and increased cellular stiffness under uniaxial cyclic stretch which agrees with experimental results. Such studies are important in exploring the differences underlying mechanotransduction and cellular contractility under stretch.








Similar content being viewed by others
References
Agrawal V, Kollimada SA, Byju AG, Gundiah N (2013) Regional variations in the nonlinearity and anisotropy of bovine aortic elastin. Biomech Model Mechanobiol 12:1181–1194
Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B (2001) Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3(5):466–472
Barreto S, Clausen CH, Perrault CM, Fletcher DA, Lacroix D (2013) A multi-structural single cell model of force-induced interactions of cytoskeletal components. Biomaterials 34(26):6119–6126
Bauër P, Tavacoli J, Pujol T, Planade J, Heuvingh J, Du Roure O (2017) A new method to measure mechanics and dynamic assembly of branched actin networks. Sci Rep 1:15688
Buck RC (1980) Reorientation response of cells to repeated stretch and recoil of the substratum. Exp Cell Res 127(2):470–474
Chen B, Kemkemer R, Deibler M, Spatz J, Gao H (2012) Cyclic stretch induces cell reorientation on substrates by destabilizing catch bonds in focal adhesions. PLoS ONE 7(11):48346
Chen Y, Pasapera AM, Koretsky AP, Waterman CM (2013) Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc Natl Acad Sci USA 110(26):E2352–E2361
Chen K, Vigliotti A, Bacca M, McMeeking RM, Deshpande VS, Holmes JW (2018) Role of boundary conditions in determining cell alignment in response to stretch. Proc Natl Acad Sci USA 115(5):986–991
Cirka H, Monterosso M, Diamantides N, Favreau J, Wen Q, Billiar K (2016) Active traction force response to long-term cyclic stretch is dependent on cell pre-stress. Biophys J 110(8):1845–1857
Clark AG, Dierkes K, Paluch EK (2013) Monitoring actin cortex thickness in live cells. Biophys J 105:570–580. https://doi.org/10.1016/j.bpj.2013.05.057
Cui Y, Hameed MF, Yang B, Lee K, Pan CQ, Park S, Sheetz M (2015) Cyclic stretching of soft substrates induces spreading and growth. Nat Commun 6(1):6333
Darling EM, Zauscher S, Block JA, Guilak FA (2007) Thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: Do cell properties reflect metastatic potential? Biophys J 92(5):1784–1791
De R, Zemel A, Safran SA (2007) Dynamics of cell orientation. Nat Phys 3(9):655–659
Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS (2002) Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J 82(5):2798–2810
Efremov YM, Velay-Lizancos M, Weaver CJ, Athamneh AI, Zavattieri PD, Suter DM, Raman A (2019) Anisotropy vs isotropy in living cell indentation with AFM. Sci Rep 9:5757
Foolen J, Marloes WJT, Broek JVD, Baaijens F (2014) Synergy between Rho signaling and matrix density in cyclic stretch-induced stress fiber organization. Acta Biomater 10:1876–1885
Gavara N, Chadwick RS (2016) Relationship between cell stiffness and stress fiber amount, assessed by simultaneous atomic force microscopy and live-cell fluorescence imaging. Biomech Model Mechanobiol 15(3):511–523
Göktepe S, Abilez OJ, Parker KK, Kuhl E (2010) A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J Theor Biol 265(3):433–442
Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R (2009) Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J Cell Sci 122(20):3644–3651
Goldyn AM, Kaiser P, Spatz JP, Ballestrem C, Kemkemer R (2010) The kinetics of force-induced cell reorganization depend on microtubules and actin. Cytoskeleton 67:241–250
Goriely A (2017) The Mathematics and mechanics of biological growth. Springer, New York. https://doi.org/10.1007/978-0-387-87710-5
Greiner AM, Chen H, Spatz JP, Kemkemer R (2013) Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0077328
Gundiah N, Ratcliffe MB, Pruitt LA (2009) The biomechanics of arterial elastin. J Mech Behav Biomed Mater 2(3):288–296
Haase K, Pelling AE (2013) The role of the actin cortex in maintaining cell shape. Commun Integr Biol 6(6):e26714
Hayakawa K, Sato N, Obinata T (2001) Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp Cell Res 268(1):104–114
Hoffman L, Jensen CC, Yoshigi M, Beckerle M (2017) Mechanical signals activate p38 MAPK pathway-dependent reinforcement of actin via mechanosensitive HspB1. Mol Biol Cell 28(20):2661–2675
Hough PVC (1962) Method and means for recognizing complex patterns. US Patent 3,069,654
Hsu HJ, Lee CF, Locke A, Vanderzyl SQ, Kaunas R (2010) Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS ONE 5(8):e12470
Huang W, Matsui ST, Saito T, Kuragano M, Takahashi M, Kawahara T, Sato M, Deguchi S (2021) Mechanosensitive myosin II but not cofilin primarily contributes to cyclic cell stretch-induced selective disassembly of actin stress fibers. Am J Physiol Cell Physiol 320:C1153–C1163
Humphrey JD, Rajagopal KR (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Model Methods Appl Sci 12(03):407–430
Imatani S, Maugin GA (2002) Constitutive model for material growth and its application to three-dimensional finite element analysis. Mech Res Commun 29(6):477–483
Jungbauer S, Gao H, Spatz JP, Kemkemer R (2008) Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J 95(7):3470–3478
Kaunas R, Nguyen P, Usami S, Chien S (2005) Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA 102(44):15895–15900
Kaunas R, Hsu HJ, Deguchi S (2010) Sarcomeric model of stretch-induced stress fiber reorganization. Cell Health Cytoskelet 3:13
Kolodney MS, Elson EL (1995) Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc Natl Acad Sci USA 92:10252–10256
Kulkarni AH, Chatterjee A, Kondaiah P, Gundiah N (2018) TGF-β induces changes in breast cancer cell deformability. Phys Biol 15(6):065005
Leccia E, Batonnet-Pichon S, Tarze A, Bailleux V, Doucet J, Pelloux M, Delort F, Pizon V, Vicart P, Briki F (2013) Cyclic stretch reveals a mechanical role for intermediate filaments in a desminopathic cell model. Phys Biol 10:016001
Lien JC, Wang YL (2021) Cyclic stretching-induced epithelial cell reorientation is driven by microtubule-modulated transverse extension during the relaxation phase. Sci Rep 11:14803
Livne A, Geiger B (2016) The inner workings of stress fibers—from contractile machinery to focal adhesions and back. J Cell Sci 129(7):1293–1304
Livne A, Bouchbinder E, Geiger B (2014) Cell reorientation under cyclic stretching. Nat Commun 5:1–8
Melnik AV, Goriely A (2013) Dynamic fiber reorientation in a fiber-reinforced hyperelastic material. Math Mech Solids 18(6):634–648
Menzel A (2005) Modelling of anisotropic growth in biological tissues. Biomech Model Mechanobiol 3(3):147–171
Merodio J, Ogden RW (2002) Material instabilities in fiber-reinforced nonlinearly elastic solids under plane deformation. Arch Mech 54(5–6):525–552
Morioka M, Parameswaran H, Naruse K, Kondo M, Sokabez M, Hesegawa Y, Suki B, Ito S (2011) Microtubule dynamics regulate cyclic stretch-induced cell alignment in human airway smooth muscle cells. PLoS ONE 6(10):e26384
Nekouzadeh A, Pryse KM, Elson EL, Genin GM (2008) Stretch-activated force shedding, force recovery, and cytoskeletal remodeling in contractile fibroblasts. J Biomech 41(14):2964–2971
Parekh SH, Chaudhuri O, Theriot JA, Fletcher DA (2005) Loading history determines the velocity of actin-network growth. Nat Cell Biol 7(12):1119–1123
Pender N, McCulloch CA (1991) Quantitation of actin polymerization in two human fibroblast sub-types responding to mechanical stretching. J Cell Sci 100:187–193
Perrault CM, Brugues A, Bazellieres E, Pierre Ricco P, Lacroix D, Trepat X (2015) Traction forces of endothelial cells under slow shear flow. Biophys J 109(8):1533–1536
Robertson AM, Watton PN (2013) Mechanobiology of the arterial wall. Transp Biol Media, pp 275–347
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4):455–467
Sakamoto Y, Buchanan RM, Sanchez-Adams J, Guilak F, Sacks MS (2017) On the functional role of valve interstitial cell stress fibers: a continuum modeling approach. J Biomech Eng 139:021007
Salbreux G, Charras G, Paluch E (2012) Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol 22:536–545
Schriefl AJ, Reinisch AJ, Sankaran S, Pierce DM, Holzapfel GA (2013) Quantitative assessment of collagen fibre orientations from two-dimensional images of soft biological tissues. J R Soc Interface 9(76):3081–3093
Thompson DW (1917) On growth and form. Cambridge University Press, Cambridge
Tjorve E, Tjorve MCK (2010) A unified approach to the Richards-model family for use in growth analyses: why we need only two model forms. J TheoR Biol 2010(267):417–425
Wang N, Stamenovic D (2000) Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol 279:188–194
Wang JHC, Goldschmidt CP, Wille J, Yin FCP (2001) Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech 34(12):1563–1572
Zielinski A, Linnartz C, Pleschka C, Dreissen G, Springer R, Merkel R, Hoffmann B (2018) Reorientation dynamics and structural interdependencies of actin, microtubules and intermediate filaments upon cyclic stretch application. Cytoskeleton 75:385–394
Preprint
Chatterjee A, Kondaiah P, Gundiah N, (2019) Stress fiber growth and remodeling determines cellular morphomechanics under uniaxial cyclic stretch, bioRxiv: https://doi.org/10.1101/622092v1. preprint posted 29 April, 2019
Acknowledgements
We thank Ms. Monisha Mohandas (BSSE, IISc) for help with the AFM. We also thank Ms. Dhulika Ravinuthala who helped in performing some of the uniaxial cyclic stretch experiments reported in this study. NG gratefully acknowledges the Department of Biotechnology (BBI2) and Department of Science and Technology (SERB/003640) for project support. PK laboratory is supported by IISc-DBT partnership program and DST-FIST infrastructure to MRDG.
Author information
Authors and Affiliations
Contributions
AC performed the experiments, analyzed the data, completed the theoretical model, and helped write the manuscript. PK provided inputs on the data interpretation and results. NG designed the study, supervised the research, helped with data analysis, and wrote the manuscript with inputs from all authors.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MOV 7303 kb)
Rights and permissions
About this article
Cite this article
Chatterjee, A., Kondaiah, P. & Gundiah, N. Stress fiber growth and remodeling determines cellular morphomechanics under uniaxial cyclic stretch. Biomech Model Mechanobiol 21, 553–567 (2022). https://doi.org/10.1007/s10237-021-01548-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01548-z