Abstract
Cellular stress and inflammation contribute to the initiation and progression of a variety of pulmonary diseases. Endoplasmic reticulum (ER) stress and its main regulator GRP78 (glucose-regulated protein 78 kDa) appear to be involved in the pathogenesis of pulmonary diseases, and GRP78 was found to be a biomarker in a wide range of inflammatory diseases. The aim of this study was to investigate the relevance of serum GRP78 in pulmonary disorders.
In this prospective cohort study, 78 consecutive patients with chronic obstructive pulmonary disease (COPD, n = 28), asthma (n = 38) or interstitial lung disease (ILD, n = 12) underwent measurement of serum GRP78 levels by ELISA.
The mean age of patients was 59.8 ± 12.4 years, 48.7% were female. Patients with elevated GRP78 levels (> median) offered a significantly better oxygenation status (capillary pO2: 75.3 ± 11.7 mmHg vs. 67.8 ± 15.9 mmHg, p = 0.02). Significant correlations were observed between GRP78, on the one hand, and haemoglobin, high-sensitivity C-reactive protein (hs-CRP) and eosinophil counts, on the other hand (haemoglobin: Pearson’s r = −0.25, hs-CRP: r = 0.30, eosinophils: r = 0.63).
Subsequently, we evaluated GRP78 measurements in function of severity stratifiers of the specific underlying pulmonary disease. ILD patients with a severe diffusion impairment (DLCO< 40% of predicted), exhibited a significant decrease in GRP78 levels (p = 0.01). In COPD and asthma, both characterized by obstructive ventilatory defects, a forced expiratory volume in one second (FEV1) <30% of predicted was accompanied by significantly lower GRP78 (p = 0.0075).
In both obstructive and restrictive pulmonary disorders, GRP78 protein concentrations were reduced with increasing disease severity. These data suggest a prevalent role of GRP78 in the presently studied pulmonary disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endoplasmic reticulum (ER) plays a crucial role in protein synthesis, correct folding and posttranslational modification of proteins (Luo & Lee, 2013). ER stress, resultant from a mismatch between ER protein folding capacity and load, leads to an accumulation of misfolded proteins in the ER (Chaudhari et al., 2014). Such an impairment in ER homeostasis exerts a major impact on cellular survival and function (Dastghaib et al., 2021). This, in turn, activates the unfolded protein response (UPR) signaling pathway in order to mitigate proteotoxic effects in stressed cells (Walter & Ron, 2011). However, in case of severe and prolonged ER stress, the UPR activates pathways that may elicit cell death through apoptosis (Wu & Kaufman, 2006).
The molecular chaperone glucose-regulated protein 78kDa (GRP78), a heat shock protein also known as binding immunoglobulin protein or heat shock protein A5, is the key mediator of the UPR (Lee, 2014). Its main function consists of restoring ER integrity via facilitating protein folding. In addition, an increasing number of recently published studies described secretion and extracellular functions of GRP78.
UPR dysregulation has been linked to numerous illnesses such as obesity, cancer and cardiovascular diseases (Dos Santos et al., 2023; Meyer & Doroudgar, 2020; Pan et al., 2022). In a study conducted by our group, we found reduced serum levels of GRP78 to predict mortality in patients with aortic valve stenosis undergoing transcatheter valve replacement (Aksoy et al., 2021). In another study, we provided evidence that elevated levels of GRP78 are linked to favourable clinical and hemodynamical parameters in patients with pulmonary arterial hypertension. Moreover, using in vitro models, we demonstrated a protective role of extracellular GRP78 in mediating pulmonary artery smooth muscle cell remodelling (Al Zaidi et al., 2022).
In chronic and debilitating respiratory disorders, the value of ER stress-UPR axis imbalances remain less well defined. Inhaled environmental stimulants, such as cigarette smoke and allergens, are known to induce ER stress and UPR dysregulation. Due to their exposition to a large number of airborne pathogens, human lungs are notably vulnerable to conditions of unresolved ER stress. A proteomics approach to biomarker in chronic obstructive pulmonary disease (COPD) identified several proteins, including GRP78, to be increased in COPD patients (Merali et al., 2014). They may reflect lung damage and airway remodelling and may potentially serve as COPD biomarkers. To assess cigarette smoke–induced lung injury, secretion of GRP78 was assayed in bronchoalveolar lavage fluid (Aksoy et al., 2017). Chronic smokers exhibited an enhanced GRP78 secretion as compared to non-smokers, supporting the notion of a GRP78 upregulation in the setting of lung oxidant stress. In idiopathic pulmonary fibrosis, emerging evidence implicates ER stress both as a cause and consequence of pulmonary inflammation and fibrosis (Dickens et al., 2019).
Given the continuous exposure of pulmonary cells to numerous environmental triggers, the aim of the present study was to investigate the potential of GRP78 as a biomarker in a cohort of patients comprising COPD, asthma and interstitial lung disease (ILD).
Methods
Study population
Between December of 2020 and April of 2021, a total of 78 consecutive patients aged ≥18 years who received treatment at the Department of Pneumology, University Hospital Bonn (Bonn, Germany), for COPD, asthma or ILD were enrolled in this prospective cohort trial. Patients were approached during their outpatient consultation, all patients presented stable disease without clinical exacerbation at the time of study inclusion. COPD diagnosis was spirometrically and clinically confirmed in line with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Global Strategy for Prevention, 2023). Asthma diagnosis complied with the Global Initiative for Asthma (GINA) diagnostic criteria (Global Initiative for Asthma: 2022 Main Report, 2023). ILD diagnosis relied on consensus by multidisciplinary discussion, according to current guidelines (Raghu et al., 2022). All patients underwent blood sampling and pulmonary function testing. Each subject completed demographic and medical history questionnaires. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the local ethics committee at the Medical Faculty of the University of Bonn. Written informed consent was obtained from all subjects prior to entry into the study.
Blood specimens
Blood samples were collected during patients’ routine outpatient visit. Samples were kept on ice and centrifugated at 1500 × g for 15 min. Subsequently, serum was transferred into coded aliquots and stored at −80°C. Analysis of GRP78 levels (Enzo Life Sciences, Inc, Farmingdale, NY, USA) was conducted by commercially available enzyme-linked immunosorbent assay (ELISA) kits. 4-Parameters Logistic Regression (Graphpad Prism 9.0.0) was applied to analyse GRP concentrations.
In addition to GRP measurement, blood samples were acquired to assess full blood count and high-sensitivity C-reactive protein (hs-CRP) levels.
Pulmonary function testing and capillary blood gas analysis
Pulmonary function testing was performed in conformity with the European Respiratory Society guidelines (Graham et al., 2019) and included spirometry, bodyplethysmography and determination of diffusion capacity for carbon monoxide (Bodyplethismograph Jaeger©, Alveo-Diffusionstest Jaeger©, Wuppertal, Germany). Static and dynamic lung volumes, such as forced expiratory volume in one second (FEV1), Tiffeneau-index (FEV1/VC) and diffusion capacity for carbon monoxide (DLCO) as assessed by the single-breath technique were analysed. Parameters were adjusted to standard population-derived predicted values.
By capillary blood gas analysis with a sample collected from the hyperaemic earlobe, partial pressure of oxygen (pO2) and partial pressure of carbon dioxide (pCO2) were determined.
Statistical analysis
Continuous data are presented as mean ± standard deviation — if normally distributed — or as median and interquartile range (IQR, quartile 1/quartile 3) — if not normally distributed. Continuous variables were tested for having a normal distribution by use of the Kolmogorov–Smirnov test. Categorical data are given as absolute numbers and percentages. In the case of continuous parameters, Student’s t-test or Mann–Whitney U-test (if normality assumption was violated) was employed for comparison between two groups. Categorical variables were analysed by Fisher´s exact test. Correlations between variables were examined with Pearson’s correlation coefficient. Statistical significance was assumed when the null hypothesis could be rejected at p < 0.05. Statistical analyses were conducted with SPSS Statistics version 26.0 (IBM, Armonk, NY, USA) and GraphPad Prism 9.0.0.
Results
Table 1 summarizes the demographic and clinical characteristics of study participants, stratified by biomarker results. Overall, patients were middle-aged (59.8 ± 12.4 years), the gender ratio was largely balanced (48.7% female). 28 out of 78 patients (35.9%) suffered from COPD, asthma was present in 38/78 patients (48.7%); the remaining 12/78 patients (15.4%) offered ILD. Intercohortal comparison as a function of the underlying pulmonary disease entity evidenced no substantial differences in GRP concentrations (Figure 1). The entire study population was stratified into two groups according to the median GRP78 plasma level of 763.6 ng/ml. Comparison of clinical data in the resultant two patient groups revealed a significantly higher capillary pO2 amongst patients with elevated GRP78 levels (75.3 ± 11.7 mmHg vs. 67.8 ± 15.9 mmHg, p = 0.02). In Table 2, pulmonary function parameters are summarized. Patients with elevated GRP78 levels had a significantly higher total lung capacity (% of predicted TLC; 114.08 ± 18.9 % vs. 103.43 ± 23.4 %, p = 0.04) and a higher residual volume (4.11 ± 1.3 L vs. 3.40 ± 1.5 L, p = 0.03). However, there was no significant difference when the residual volume was analysed as a percentage of predicted volume.
Statistically significant correlations were observed between GRP78 concentrations, on the one hand, and haemoglobin, hs-CRP and eosinophil counts, on the other hand (haemoglobin: Pearson’s r = −0.25, p = 0.03; hs-CRP: Pearson’s r = 0.30, p = 0.01; eosinophils: Pearson’s r = 0.63, p < 0.0001; Figure 2). By contrast, total leukocyte count was not related to GRP78 plasma level (Pearson’s r = 0.11, p = 0.36).
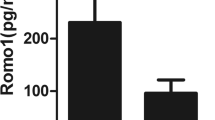
Subsequently, we evaluated GRP78 measurements in function of severity stratifiers of the specific underlying pulmonary disease. In terms of diffusion capacity, a DLCO below 40% of the predicted value discriminates between severe and moderate diffusion reduction and is an established definer of advanced ILD. In our ILD cohort, patients with a DLCO <40% of predicted exhibited a significant decrease in GRP78 levels (524 ng/ml (226-656) vs. 1126 ng/ml (647–1701), p = 0.01; Figure 3). In ILD patients, GRP78 levels positively correlated with the DLCO (Pearson’s r = 0.58, p = 0.048).
In COPD and asthma, both disease entities characterized by obstructive ventilatory defects, a FEV1 <30% of the predicted value marks severe obstructive airflow limitation (Global Strategy for Prevention, 2023; Global Initiative for Asthma: 2022 Main Report, 2023). When transferred to our COPD and asthma study population, significantly lower GRP78 levels were measured in patients with a FEV1 <30% of predicted (p = 0.0075, Figure 4).
With a view to symptom burden, the COPD Assessment Test (CAT) and the Asthma Control Test (ACT) were performed, both validated questionnaires to evaluate symptomatology in COPD and asthma, respectively. A CAT-score ≥10 and an ACT-score <16 indicate lacking disease control. As to our asthma cohort, symptom burden showed no association with GRP78 measurements (p = 0.35, Figure 5). Regarding our COPD cohort, all patients included into the study stated poor disease control (CAT-score ≥10), irrespective of GRP78-levels.
Discussion
In the present study, we aimed to determine the potential of serum GRP78 as a biomarker in chronic pulmonary diseases encompassing COPD, asthma and ILD. The main findings of our study are as follows: (i) Increased GRP78 concentrations were accompanied by improved oxygenation status. (ii) In terms of severity of the specific underlying disease entity, decreased GRP78 levels were associated with severity of obstructive airflow limitation in COPD and asthma, on the one hand, and with severity of diffusion capacity limitation in ILD, on the other hand. In light of this association between elevated GRP78 protein expression and a favourable disease course, a protective effect of GRP78 on pulmonary disorders is presumable.
Given its vast surface area of approximately 100 m2 and the high volume of inhaled air, the lung is continuously exposed to noxae including environmental pollutants, cigarette smoke, pathogens and allergens. Accumulating evidence suggests that these constant environmental threats may elicit protein misfolding and subsequently activate the UPR signaling pathway (Osorio et al., 2013). COPD constitutes a chronic, debilitating pulmonary disorder of multicomponent nature, defined by chronic airway inflammation (Aksoy et al., 2017). This inflammatory response, resultant from exposure to noxious fumes and airborne particles, is noticeable at both a local and systemic level (Oudijk et al., 2003). Recently, considerable efforts have been made to clarify the role of the ER stress-UPR axis in COPD (Barreiro et al., 2019; Naiel et al., 2020). Airway cell damage in COPD is mainly attributable to oxidative stress, originated by inhaled environmental triggers. Oxidants (e.g., reactive oxygen species) mediate oxidative stress response, leading to activation of the UPR pathway (Aghaei et al., 2020). However, the precise downstream regulatory interactions that allow for recognition and handling of these inhaled noxae remain undefined. Enhanced concentrations of GRP78, a major player in the UPR, has been ascertained in the bronchoalveolar lavage fluid and lung specimens of active smokers (Aksoy et al., 2017; Kelsen et al., 2008). In COPD, GRP78 has been reported as a potential biomarker, correlating with both FEV1 and the extent of emphysema (Merali et al., 2014). Similarly, we presently observed altered GRP78 concentrations dependent on the severity of the underlying obstructive pulmonary disorder. In case of severe airflow obstruction, defined by a FEV1 <30% of the predicted value, GRP78 was significantly reduced. This observation is in line with the assumption that increased GRP78 expression mitigates ER stress-induced lung inflammation and apoptosis and thereby exhibits protective effects in inflammatory pulmonary diseases.
Beyond COPD, asthma represents the second most frequent obstructive respiratory disorder, likewise characterized by chronic airway inflammation. As a function of the underlying disease processes that determine clinical and pathophysiological features, a distinction is made between diverse asthma phenotypes (Global Initiative for Asthma: 2022 Main Report, 2023). They encompass the broad range of allergic, eosinophilic and neutrophilic asthma. Allergic asthma is defined by the induction of IgE antibodies subsequent to allergen exposition. Most elicitors of allergic asthma are capable of inducing ER stress-UPR response. Pathinayake and colleagues demonstrated evidence of ER stress/UPR in induced sputum and bronchoscopy specimens of patients with asthma (Pathinayake et al., 2022). ER stress was mainly present in those patients with active eosinophilic and neutrophilic airway inflammation. Moreover, GRP78 gene expression was associated with lung function decline. Consistently, we measured lowest GRP78 levels in patients with severe airway obstruction, as assessed by pulmonary function testing. Remarkably, the positive correlation observed between GRP78 and eosinophil counts in the entire patient population was solely driven by the asthma cohort (Pearson’s r = 0.79, p < 0.001). To address the role of eosinophils in allergic inflammation, Wang et al. challenged eosinophil-deficient mice with Aspergillus fumigatus, resulting in fungus-induced allergic lung inflammation (Wang et al., 2021). They ascertained decreased GRP78 protein levels in bronchial epithelial cells of fungus-exposed, eosinophil-deficient mice as compared to fungus-exposed, eosinophil-sufficient wild-type mice. Additionally, at a lung gene level, GRP78 was upregulated in challenged wild-type mice. Increased apoptosis was found in both challenged eosinophil-deficient and -sufficient mice, though the latter displayed a milder apoptotic cell activity. These findings support the notion that eosinophils promote an upregulation of the UPR pathway and are detrimental in allergic pulmonary response. Notwithstanding, the question whether UPR activation negatively impacts cellular survival or rather intends restoration of cellular damage control in eosinophilic allergic lung inflammation remains to be answered.
ILD encompasses a large group of more than 200 parenchymal lung diseases that share similar clinical, radiological and pathophysiological characteristics (Travis et al., 2013). A subset of ILD patients may develop progressive pulmonary fibrosis. Idiopathic pulmonary fibrosis (IPF) represents the archetypal progressive fibrotic ILD characterized by relentless lung function decline and earlier mortality (Raghu et al., 2022). In terms of ER stress and UPR activation in ILD, most evidence exists for IPF. Increased ER stress-UPR response including enhanced GRP78 expression has been found in the alveolar epithelium of IPF patients (Lawson et al., 2008). However, UPR signature is not limited to the alveolar epithelium, but also prominent in subjacent fibroblast foci, supporting a correlation between ER stress and IPF pathogenesis. Exogenous stimulants known to increase the probability of developing IPF in genetically susceptible individuals, comprise cigarette smoke, viral infections, microaspiration, airborne pollutants and occupational exposures (Wolters et al., 2018). Noteworthy, many of these risk factors are established ER stressors, indicating that ER stress and UPR appear to be critically involved in IPF onset and progression. Within our ILD study population, only two patients presented IPF. The remaining ILD study cohort was heterogenous with regard to the underlying ILD aetiology but shared the similarity of being mostly progressive fibrosing in nature. In ILD patients, GRP78 levels positively correlated with DLCO (Pearson’s r = 0.58, p = 0.048). This association was particularly noticeable in those patients with severe diffusion capacity impairment, defined by a DLCO <40% of the predicted value. To the best of our knowledge, no data exist by now evaluating the relation between pulmonary function decline in ILD and chaperone levels. Diffusion capacity represents an established physiological marker of disease severity and progression in ILD. Our finding of a positive correlation between GRP78 and diffusion capacity is consistent with our aforementioned results in obstructive ventilatory defects, in which disease severity is mainly defined by the degree of obstructive airflow limitation, quantified by FEV1. In both obstructive and restrictive pulmonary disorders, GRP78 protein concentrations were reduced with increasing disease severity. In keeping with this, we ascertained the highest GRP78 levels in patients with best oxygenation status. While clearly, we cannot address the mechanisms underlying this suggestion, these data enhance the idea that GRP78 may exert protective effects on the course of chronic inflammatory pulmonary diseases like COPD, asthma or ILD.
Our study has several limitations including its single-centre design that impeded the inclusion of a larger number of patients. The study population was composed of patients exhibiting three distinct chronic pulmonary illnesses. Moreover, within the ILD patient cohort, heterogeneity arose from the varying ILD-underlying aetiologies. However, these aetiological variations were potentially overcome by the commonality of a progressive fibrosing conduct. Finally, we confined GRP78 association analyses to pulmonary function and biochemical parameters. Additional chest imaging might have been a valuable adjunct to determine the radiological extent of disease severity.
Our results suggest that GRP78 could be clinically utilized as an additional marker for performing risk stratification in patients with pulmonary diseases. Patients with low GRP78 levels might benefit from closer supervision to detect clinical deterioration. Additionally, we plan to conduct larger follow-up studies to examine prognostic properties of GRP78 in patients with pulmonary disorders. These results will provide further evidence to the significance of GRP78 as biomarker in patients with lung diseases.
In conclusion, the present study implicates GRP78, a hallmark effector of the response to ER stress, in the progression of chronic pulmonary diseases. Elevated GRP78 levels were associated with a more favourable disease course. Taken together, these data support the concept of a presumably protective role of GRP78 in the presently studied pulmonary disorders. Simultaneously, they set the stage for larger studies including disease follow-up.
References
Aghaei M, Dastghaib S, Aftabi S, Aghanoori MR, Alizadeh J, Mokarram P, Mehrbod P, Ashrafizadeh M, Zarrabi A, McAlinden KD, Eapen MS, Sohal SS, Sharma P, Zeki AA, Ghavami S (2020) The ER stress/UPR axis in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Life 11(1):1
Aksoy A, Al-Kassou B, Al Zaidi M, Shamekhi J, Repges E, Sedaghat A, Becher MU, Treede H, Jansen F, Sinning JM, Zimmer S, Nickenig G, Tiyerili V (2021) Circulating chaperones in patients with aortic valve stenosis undergoing TAVR: impact of concomitant chronic kidney disease. Transl Res 233:117–126
Aksoy MO, Kim V, Cornwell WD, Rogers TJ, Kosmider B, Bahmed K, Barrero C, Merali S, Shetty N, Kelsen SG (2017) Secretion of the endoplasmic reticulum stress protein, GRP78, into the BALF is increased in cigarette smokers. Respir Res 18(1):78
Al Zaidi M, Pizarro C, Bley C, Repges E, Sedaghat A, Zimmer S, Jansen F, Tiyerili V, Nickenig G, Skowasch D, Aksoy A (2022) ER-stress-induced secretion of circulating glucose-regulated protein 78kDa (GRP78) ameliorates pulmonary artery smooth muscle cell remodelling. Cell Stress Chaperones 27:561–572
Barreiro E, Salazar-Degracia A, Sancho-Muñoz A, Aguiló R, Rodríguez-Fuster A, Gea J (2019) Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dysfunction of patients with stable chronic obstructive pulmonary disease. J Appl Physiol 126(6):1572–1586
Chaudhari N, Talwar P, Parimisetty A, Lefebvre d'Hellencourt C, Ravanan P (2014) A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci 8:213
Dastghaib S, Kumar PS, Aftabi S, Damera G, Dalvand A, Sepanjnia A, Kiumarsi M, Aghanoori MR, Sohal SS, Ande SR, Alizadeh J, Mokarram P, Ghavami S, Sharma P, Zeki AA (2021) Mechanisms targeting the unfolded protein response in asthma. Am J Respir Cell Mol Biol 64(1):29–38
Dickens JA, Malzer E, Chambers JE, Marciniak SJ (2019) Pulmonary endoplasmic reticulum stress-scars, smoke, and suffocation. FEBS J 286(2):322–341
Dos Santos NS, Gonçalves DR, Balbinot B, Visioli F (2023) Is GRP78 (Glucose-regulated protein 78) a prognostic biomarker in differents types of cancer? A systematic review and meta-analysis. Pathol Res Pract 242:154301
Global Initiative for Asthma: 2022 Main Report. 2023, https://ginasthma.org/gina-reports.
Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. 2023, https://goldcopd.org/2023-gold-report-2.
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, Oropez CE (2019) Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 200:e70–e88
Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S (2008) Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol 38(5):541–550
Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS (2008) Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294(6):L1119–L1126
Lee AS (2014) Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer 14(4):263–276
Luo B, Lee AS (2013) The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 32(7):805–818
Merali S, Barrero CA, Bowler RP, Chen DE, Criner G, Braverman A, Litwin S, Yeung A, Kelsen SG (2014) Analysis of the plasma proteome in COPD: novel low abundance proteins reflect the severity of lung remodeling. COPD 11(2):177–189
Meyer BA, Doroudgar S (2020) ER stress-induced secretion of proteins and their extracellular functions in the heart. Cells 9(9):2066
Naiel S, Tat V, Padwal M, Vierhout M, Mekhael O, Yousof T, Ayoub A, Abed S, Dvorkin-Gheva A, Ask K (2020) Protein misfolding and endoplasmic reticulum stress in chronic lung disease: will cell-specific targeting be the key to the cure? Chest 157(5):1207–1220
Osorio F, Lambrecht B, Janssens S (2013) The UPR and lung disease. Semin Immunopathol 35(3):293–306. https://doi.org/10.1007/s00281-013-0368-6
Oudijk EJ, Lammers JW, Koenderman L (2003) Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J Suppl 46:5s–13s
Pan D, Yang Y, Nong A, Tang Z, Li QX (2022) GRP78 activity moderation as a therapeutic treatment against obesity. Int J Environ Res Public Health 19(23):15965
Pathinayake PS, Waters DW, Nichol KS, Brown AC, Reid AT, Hsu AC, Horvat JC, Wood LG, Baines KJ, Simpson JL, Gibson PG, Hansbro PM, Wark PAB (2022) Endoplasmic reticulum-unfolded protein response signalling is altered in severe eosinophilic and neutrophilic asthma. Thorax 77(5):443–451
Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM, Martinez FJ, Molina-Molina M, Myers JL, Nicholson AG, Ryerson CJ, Strek ME, Troy LK, Wijsenbeek M, Mammen MJ, Hossain T et al (2022) Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 205(9):e18–e47
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M et al (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188(6):733–748
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Wang S, Jiang Z, Li L, Zhang J, Zhang C, Shao C (2021) Ameliorative effects of eosinophil deficiency on immune response, endoplasmic reticulum stress, apoptosis, and autophagy in fungus-induced allergic lung inflammation. Respir Res 22(1):173
Wolters PJ, Blackwell TS, Eickelberg O, Loyd JE, Kaminski N, Jenkins G, Maher TM, Molina-Molina M, Noble PW, Raghu G, Richeldi L, Schwarz MI, Selman M, Wuyts WA, Schwartz DA (2018) Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med 6(2):154–160
Wu J, Kaufman RJ (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13(3):374–384
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. This work was supported by the medical faculty of the University of Bonn (BONFOR Grant No. 2018-1A-02 and 2021-2A-04 to AA).
Author information
Authors and Affiliations
Contributions
The authors Muntadher Al Zaidi, Vanessa Marggraf, Adem Aksoy and Carmen Pizarro contributed equally.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Ethical approval was granted from the Ethics Committee of the University of Bonn. Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Zaidi, M., Marggraf, V., Repges, E. et al. Relevance of serum levels of the endoplasmic reticulum stress protein GRP78 (glucose-regulated protein 78 kDa) as biomarker in pulmonary diseases. Cell Stress and Chaperones 28, 333–341 (2023). https://doi.org/10.1007/s12192-023-01341-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-023-01341-0