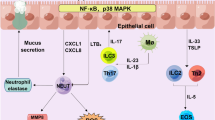
Abstract
The respiratory tract has a surface area of approximately 70 m2 that is in direct contact with the external environment. Approximately 12,000 l of air are inhaled daily, exposing the airway epithelium to up to 25 million particles an hour. Several inhaled environmental triggers, like cigarette smoke, diesel exhaust, or allergens, are known inducers of endoplasmatic reticulum (ER) stress and cause a dysregulation in ER homeostasis. Furthermore, some epithelial cell types along the respiratory tract have a secretory function, producing large amounts of mucus or pulmonary surfactant, as well as innate host defense molecules like defensins. To keep up with their secretory demands, these cells must rely on the appropriate functioning and folding capacity of the ER, and they are particularly more vulnerable to conditions of unresolved ER stress. In the lung interstitium, triggering of ER stress pathways has a major impact on the functioning of vascular smooth muscle cells and fibroblasts, causing aberrant dedifferentiation and proliferation. Given the large amounts of foreign material inhaled, the lung is densely populated by various types of immune cells specialized in engulfing and killing pathogens and in secreting cytokines/chemokines for efficient microbial clearance. Unfolded protein response signaling cascades have been shown to intersect with the functioning of immune cells at all levels. The current review aims to highlight the role of ER stress in health and disease in the lung, focusing on its impact on different structural and inflammatory cell types.




Similar content being viewed by others
Abbreviations
- AEC:
-
Alveolar epithelial cell
- AGR2:
-
Anterior gradient homolog 2
- ATF6:
-
Activating transcription factor 6
- CHOP:
-
CCAAT/enhancer-binding protein homologous protein
- CF:
-
Cystic fibrosis
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- ChIP:
-
Chromatin immunoprecipitation
- CREB:
-
cAMP response element binding
- COPD:
-
Chronic obstructive pulmonary disease
- CRT:
-
Calreticulin
- CS:
-
Cigarette smoke
- DCs:
-
Dendritic cells
- ECs:
-
Epithelial cells
- eIF2a:
-
Elongation initiation factor 2α
- ER:
-
Endoplasmatic reticulum
- ERAD:
-
ER-associated degradation
- Foxo2a:
-
Forkhead box-family transcription factor 2a
- GASML:
-
Gasdermin B
- GCM:
-
Goblet cell metaplasia
- GCN2:
-
General control nonrepressed
- Grp78:
-
Glucose regulated protein 78
- HDM:
-
House dust mite
- IPF:
-
Idiopathic pulmonary fibrosis
- IIPs:
-
Idiopathic interstitial pneumonias
- IRE1:
-
Inositol requiring enzyme 1
- JNK:
-
c-Jun N-terminal kinase
- NO:
-
Nitric oxide
- Nrf-2:
-
Nuclear factor erythroid 2-related factor
- ORMDL3:
-
Orosomucoid-like 3
- PAH:
-
Pulmonary arterial hypertension
- PDI:
-
Protein disulfide isomerase
- PERK:
-
Protein kinase R-like ER kinase
- PM:
-
Particulate matter
- RIDD:
-
Regulated IRE1-dependent decay
- ROS:
-
Reactive oxygen species
- S1P:
-
Sphingosine-1 phosphate
- SERCA:
-
Sarco-endoplasmic reticulum Ca2+ ATPase pump
- SMC:
-
Smooth muscle cells
- SPDEF:
-
SAM pointed domain containing Ets
- SPC:
-
Surfactant protein C
- TGF-β:
-
Transforming growth factor β
- TLR4:
-
Toll-like receptor 4
- UPR:
-
Unfolded protein response
- XBP1:
-
X-box binding protein 1
References
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102
Bertolotti A et al (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2(6):326–32
Credle JJ et al (2005) On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci U S A 102(52):18773–84
Kawaguchi S, Ng DT (2011) Cell biology.Sensing ER stress. Science 333(6051):1830–1
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–29
Urano F et al (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–6
Zhou Y et al (2011) Regulation of glucose homeostasis through a XBP-1–FoxO1 interaction. Nat Med 17(3):356–365
Shaffer AL et al (2004) XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21(1):81–93
Lee A-H et al (2008) Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320(5882):1492–1496
Acosta-Alvear D et al (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27(1):53–66
Hollien J, Weissman JS (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313(5783):104–107
Hollien J et al (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186(3):323–331
Hur KY et al (2012) IRE1α activation protects mice against acetaminophen-induced hepatotoxicity. J Exp Med 209(2):307–318
Asada R et al (2011) The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem 149(5):507–18
Wu J et al (2007) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13(3):351–64
Kondo S et al (2012) Activation of OASIS family, ER stress transducers, is dependent on its stabilization. Cell Death Differ 19:1939–1949
Asada R et al (2012) The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J Biol Chem 287(11):8144–53
Harding HP et al (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5(5):897–904
Harding HP et al (2001) Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7(6):1153–63
Cullinan SB et al (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23(20):7198–7209
Chan K, Kan YW (1999) Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A 96(22):12731–6
Iwakoshi NN et al (2003) Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol 4(4):321–329
Iwakoshi NN, Pypaert M, Glimcher LH (2007) The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med 204(10):2267–2275
Tohmonda T et al (2011) The IRE1alpha–XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep 12(5):451–7
Lambrecht BN, Hammad H (2012) The airway epithelium in asthma. Nat Med 18(5):684–92
Park KS et al (2007) SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 117(4):978–88
Park KS et al (2006) Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 34(2):151–7
Ribeiro CM, O'Neal WK (2012) Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr Mol Med 12(7):872–82
Ribeiro CMP, Boucher RC (2010) Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc Am Thorac Soc 7(6):387–394
Martino MEB et al (2009) Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem 284(22):14904–14913
Kaser A et al (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134(5):743–756
Heazlewood CK et al (2008) Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 5(3):e54
Eri RD et al (2011) An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol 4(3):354–364
Martino MB et al (2012) The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. doi:10.1038/mi.2012.105
Bertolotti A et al (2001) Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest 107(5):585–593
Calfon M et al (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415(6867):92–96
Imagawa Y et al (2008) RNase domains determine the functional difference between IRE1alpha and IRE1beta. FEBS Lett 582(5):656–660
Iqbal J et al (2008) IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab 7(5):445–55
Iwawaki T et al (2001) Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol 3(2):158–164
Rogers DF (2007) Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care 52(9):1134–46, discussion 1146–9
Park S-W et al (2009) The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A 106(17):6950–6955
Zhao F et al (2010) Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol 338(2):270–279
Zheng W et al (2006) Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun 7(1):11–8
Whitsett JA, Weaver TE (2002) Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 347(26):2141–8
Wang Y et al (2009) Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 84(1):52–9
Bullard JE et al (2005) ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med 172(8):1026–31
King TE Jr, Pardo A, Selman M (2011) Idiopathic pulmonary fibrosis. Lancet 378(9807):1949–61
Korfei M et al (2008) Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178(8):838–46
Maguire JA, Mulugeta S, Beers MF (2012) Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by Surfactant Protein C BRICHOS mutants. Int J Biochem Cell Biol 44(1):101–12
Weichert N et al (2011) Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res 12:4
Franke-Ullmann G et al (1996) Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immun (Baltimore, Md: 1950) 157(7):3097–3104
Martinon F et al (2010) TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Publishing Group 11(5):411–418
Woo CW et al (2012) Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat Cell Biol 14(2):192–200
Plantinga M et al (2013) Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38:322–335
McGill J, van Rooijen N, Legge KL (2008) Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med 205(7):1635–1646
GeurtsvanKessel CH et al (2008) Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J Exp Med 205(7):1621–1634
Lambrecht BN, Hammad H (2012) Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol 30:243–270
Hu F et al (2011) ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. Eur J Immunol 41(4):1086–1097
Goodall JC et al (2010) Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A 107(41):17698–17703
Ulianich L et al (2011) ER stress impairs MHC Class I surface expression and increases susceptibility of thyroid cells to NK-mediated cytotoxicity. Biochim Biophys Acta 1812(4):431–438
Granados DP et al (2009) ER stress affects processing of MHC class I-associated peptides. BMC Immunol 10:10
Reimold AM et al (1996) Transcription factor B cell lineage-specific activator protein regulates the gene for human X-box binding protein 1. J Exp Med 183(2):393–401
Peters LR, Raghavan M (2011) Endoplasmic reticulum calcium depletion impacts chaperone secretion, innate immunity, and phagocytic uptake of cells. J Immunol 187(2):919–931
Panaretakis T et al (2009) Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 28(5):578–590
Garg AD et al (2012) A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 31(5):1062–1079
Obeid M et al (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13(1):54–61
Todd DJ, Lee A-H, Glimcher LH (2008) The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 8(9):663–674
Hengstermann A, Müller T (2008) Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Radic Biol Med 44(6):1097–1107
Knörr-Wittmann C et al (2005) Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radic Biol Med 39(11):1438–1448
Tagawa Y et al (2008) Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP). Free Radic Biol Med 45(1):50–59
Jorgensen E et al (2008) Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 8:229
Kelsen SG et al (2007) Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol 38(5):541–550
Malhotra D et al (2009) Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med 180(12):1196–1207
Saxon A, Diaz-Sanchez D (2005) Air pollution and allergy: you are what you breathe. Nat Immunol 6(3):223–226
Brunekreef B, Holgate ST (2002) Air pollution and health. Lancet 360(9341):1233–1242
Laing S et al (2010) Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol 299(4):C736–49
Li YJ et al (2008) Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol (Orlando, Fla) 128(3):366–373
Palm NW, Rosenstein RK, Medzhitov R (2012) Allergic host defences. Nature 484(7395):465–472
Trompette A et al (2009) Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457(7229):585–588
Wang S, Kaufman RJ (2012) The impact of the unfolded protein response on human disease. J Cell Biol 197(7):857–867
Hammad H, Lambrecht BN (2012) The airway epithelium in asthma. Nat Med 18(5):684–692
He B (2006) Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ 13(3):393–403
Roberson EC et al (2012) Influenza induces endoplasmic reticulum stress, caspase-12-dependent apoptosis, and c-Jun N-terminal kinase-mediated transforming growth factor-β release in lung epithelial cells. Am J Respir Cell Mol Biol 46(5):573–581
Hassan IH et al (2012) Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J Biol Chem 287(7):4679–4689
Smith JA et al (2008) Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur J Immunol 38(5):1194–1203
Zeng L et al (2010) XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol 185(4):2324–2330
Hammad H et al (2009) House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 15(4):410–416
Kuperman DA et al (2005) Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol 116(2):305–311
Schroeder BW et al (2012) AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol 47(2):178–185
Chen G et al (2010) Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J Immunol 184(11):6133–41
Rangasamy T et al (2005) Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202(1):47–59
Belmont PJ et al (2012) Regulation of microRNA expression in the heart by the ATF6 branch of the ER stress response. J Mol Cell Cardiol 52:1176–1182
Voeltz GK et al (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124(3):573–586
Wright PL et al (2010) Epithelial reticulon 4B (Nogo-B) is an endogenous regulator of Th2-driven lung inflammation. J Exp Med 207(12):2595–2607
Moffatt MF et al (2007) Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 448(7152):470–473
Bouzigon E et al (2008) Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med 359(19):1985–1994
Galanter J et al (2008) ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med 177(11):1194–1200
Hirota T et al (2008) Genetic polymorphism regulating ORM1-like 3 (Saccharomyces cerevisiae) expression is associated with childhood atopic asthma in a Japanese population. J Allergy Clin Immunol 121(3):769–770
Hjelmqvist L et al (2002) ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biol 3(6):RESEARCH0027
Han S et al (2010) Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc Natl Acad Sci U S A 107(13):5851–5856
Cantero-Recasens G et al (2010) The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet 19(1):111–121
Breslow DK et al (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463(7284):1048–1053
Idzko M et al (2006) Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest 116(11):2935–44
Ryan JJ, Spiegel S (2008) The role of sphingosine-1-phosphate and its receptors in asthma. Drug News Perspect 21(2):89–96
Verlaan DJ et al (2009) Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am J Hum Genet 85(3):377–393
Miller M et al (2012) ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci U S A 109:16648–16653
Wynn TA (2011) Integrating mechanisms of pulmonary fibrosis. J Exp Med 208(7):1339–50
Zoz DF, Lawson WE, Blackwell TS (2011) Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci 341(6):435–8
Selman M, Pardo A (2002) Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res 3:3
Uhal BD et al (1998) Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 275(6 Pt 1):L1192–9
Sisson TH et al (2010) Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181(3):254–63
Kuwano K et al (1999) Essential roles of the Fas–Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 104(1):13–9
Tanjore H, Blackwell TS, Lawson WE (2012) Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 302(8):L721–9
Lawson WE et al (2011) Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A 108(26):10562–10567
Tang YW et al (2003) Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 41(6):2633–40
Kropski JA, Lawson WE, Blackwell TS (2012) Right place, right time: the evolving role of herpesvirus infection as a "second hit" in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 302(5):L441–4
Lawson WE et al (2008) Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294(6):L1119–26
Rock JR et al (2011) Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A 108(52):E1475–83
Tanjore H et al (2011) Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem 286(35):30972–30980
Zhong Q et al (2011) Role of endoplasmic reticulum stress in epithelial–mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol 45(3):498–509
Tsang KY et al (2007) Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol 5(3):e44
Baek HA et al (2012) Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol 46(6):731–9
Rabinovitch M (2008) Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118(7):2372–9
Giaid A et al (1993) Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328(24):1732–9
Giaid A, Saleh D (1995) Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333(4):214–21
Macchia A et al (2010) Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J 159(2):245–57
Sutendra G et al (2010) Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med 2(44):44ra58
Xu W et al (2007) Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A 104(4):1342–7
McMurtry MS et al (2004) Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95(8):830–40
Sutendra G et al (2011) The role of Nogo and the mitochondria–endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med 3(88):88ra55
Yeager ME et al (2012) Endothelin-1, the unfolded protein response, and persistent inflammation: role of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 46(1):14–22
Yeager ME et al (2012) Activation of the unfolded protein response is associated with pulmonary hypertension. Pulm Circ 2(2):229–40
Lenna S et al (2010) HLA-B35 upregulates endothelin-1 and downregulates endothelial nitric oxide synthase via endoplasmic reticulum stress response in endothelial cells. J Immunol 184(9):4654–61
Acevedo L et al (2004) A new role for Nogo as a regulator of vascular remodeling. Nat Med 10(4):382–8
Chipuk JE et al (2012) Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148(5):988–1000
Gilbert A et al (1998) Delta F508 CFTR localizes in the endoplasmic reticulum–Golgi intermediate compartment in cystic fibrosis cells. Exp Cell Res 242(1):144–52
Davis PB, Drumm M, Konstan MW (1996) Cystic fibrosis. Am J Respir Crit Care Med 154(5):1229–56
Hollenhorst MI, Richter K, Fronius M (2011) Ion transport by pulmonary epithelia. J Biomed Biotechnol 2011:174306
Matsui H et al (1998) Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95(7):1005–15
Doring G, Gulbins E (2009) Cystic fibrosis and innate immunity: how chloride channel mutations provoke lung disease. Cell Microbiol 11(2):208–16
Zahm JM et al (1997) Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am J Physiol 272(3 Pt 1):C853–9
Tirouvanziam R et al (2000) Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol 23(2):121–7
Kerbiriou M et al (2007) Coupling cystic fibrosis to endoplasmic reticulum stress: differential role of Grp78 and ATF6. Biochim Biophys Acta 1772(11–12):1236–49
Bartoszewski R et al (2008) The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J Biol Chem 283(18):12154–65
Bartoszewski R et al (2008) Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol 39(4):448–57
Rab A et al (2007) Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am J Physiol Cell Physiol 292(2):C756–66
Weber AJ et al (2001) Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl− channel function. Am J Physiol Lung Cell Mol Physiol 281(1):L71–8
Ribeiro CMP et al (2005) Chronic airway infection/inflammation induces a Ca2+ i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem 280(18):17798–17806
Teichgraber V et al (2008) Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 14(4):382–91
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on “The unfolded protein response in immune diseases”—Guest Editors: Richard Blumberg and Arthur Kaser
Rights and permissions
About this article
Cite this article
Osorio, F., Lambrecht, B. & Janssens, S. The UPR and lung disease. Semin Immunopathol 35, 293–306 (2013). https://doi.org/10.1007/s00281-013-0368-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-013-0368-6