Abstract
This study aimed to elucidate the molecular mechanisms of hypoxia/reoxygenation (H/R) injury in human cardiac microvascular endothelial cells (HCMECs) by regulating ferroptosis. H/R model was established with HCMECs and before the reperfusion, ferroptosis inhibitor ferrostatin-1 or ferroptosis inducer erastin was all administered. Wound-healing assay was performed to detect the migration ability of cells in each group, and the angiogenesis ability was determined by tube formation assay. The level of reactive oxygen species (ROS) was detected by flow cytometry. Transmission electron microscopy (TEM) was used to observe the state of mitochondria. The expressions of related proteins in HCMECs were assessed by Western blot. From the results, H/R injury could inhibit the migration and angiogenesis, induce the ROS production, and cause the mitochondrial damage of HCMECs. Ferroptosis activator erastin could aggravate H/R injury in HCMECs, while the ferroptosis inhibitor ferrostatin-1 could reverse the effects of H/R on HCMECs. Western blot results showed that H/R or/and erastin treatment could significantly induce ACSL4, HGF, VEGF, p-ERK, and uPA protein expression and inhibit GPX4 expression. The addition of ferrostatin-1 resulted in the opposite trend of the proteins expression above to erastin treatment. What is more, overexpression of ENPP2 markedly suppressed the damaging effect of H/R on HCMECs and reversed the effects of H/R or erastin treatment on the expression of related proteins. These results demonstrated a great therapeutic efficacy of ENPP2 overexpression in preventing the development of H/R injury through inhibiting oxidative stress and ferroptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial ischemia refers to the pathophysiological state of abnormal myocardial energy metabolism due to insufficient blood supply and oxygen supply, resulting in the failure of the heart to maintain its normal function (Ambrose 2006). The incidence rate of myocardial ischemia accounts for the highest proportion in many pathogenesis associated with heart failure, which poses a great health threat to the patients with cardiovascular disease (Pagliaro, Cannata, Stefanini, and Bolognese 2020). At present, coronary artery reperfusion therapy is mainly used in clinic to effectively reduce myocardial ischemia injury and prevent further tissue damage, but the reperfusion process will also bring damage that cannot be ignored (Binder et al. 2015; B. Shi, Ma, Zheng 2019, Pan, and Lin 2019). Myocardial ischemia/reperfusion (I/R) is a pathological process characterized by insufficient oxygen supply and subsequent blood flow recovery that occurs in many organs and diseases with high morbidity and mortality (Zhang et al. 2022). I/R injury may be caused by pathological processes and/or secondary to surgical processes, and the original I/R injury is more severe (B. Shi et al. 2019). Additionally, the mechanisms contributing to the pathogenesis of I/R is complex, multifactorial, and highly integrated (Lejay et al. 2016). Consequently, preventing hypoxia- reoxygenation (H/R) injury and resolving its molecular mechanism are vital for improving the healing rate of myocardial I/R injury.
Cardiac microvascular endothelial cells (CMECs) are, by necessity, the cells affected in the cardiac microcirculation by I/R injury (Cui et al. 2016). Compared to vascular endothelial cells, CMECs are more sensitive to ischemic injury and have attracted much attention in preventing myocardial I/R injury (Singhal, Symons, Boudina, Jaishy, and Shiu 2010). Previous studies have shown that diversified abnormal responses and detrimental changes of CMECs were initiated by I/R, including excessive inflammation, reactive oxygen species (ROS) production and apoptosis, contributing to the progression of cardiac dysfunction (Y. Liu et al. 2014; Zhu et al. 2018).
Recent studies have demonstrated that ferroptosis and oxidative stress ferroptosis are closely related to the process of I/R injury. Oxidative stress is usually caused by uncontrolled high levels of ROS, resulting in imbalance of oxidation and antioxidant systems and cell and tissue damage (García, Zazueta, and Aguilera-Aguirre 2017; González-Montero, Brito, Gajardo, and Rodrigo 2018; Kandula et al. 2016). Since ROS induces multiple types of cell death, including apoptosis, necrosis, and pyroptosis, it is considered to have a major role in I/R injury of many organs (Li et al. 2018). Ferroptosis is an iron-dependent regulatory of cell death (Dixon et al. 2012), which has been found to have a role in many diseases and has been proposed as a new therapeutic strategy for I/R injury (She, Lan, Tian, and Tang 2020). There is evidence that inhibiting ferroptosis had the ability of alleviating diabetes I/R injury, which might give a therapeutic direction for myocardial ischemic disease (W. Li et al. 2020). Importantly, it is currently believed that ferroptosis is mainly related to abnormal toxicity of iron, lipid peroxidation, and mitochondrial dysfunction (Conrad et al. 2018). Moreover, researchers found that selenium can reduce cerebral I/R injury through affecting oxidative stress, ferroptosis, and mitochondrial fusion in vivo and in vitro (Y. Shi et al. 2022). However, whether myocardial I/R regulates human cardiac microvascular endothelial cell (HCMEC) injury through ferroptosis and the specific regulatory mechanism are still unclear.
Here, to address these unanswered questions, we constructed hypoxia/reoxygenation (H/R) cell model in vitro with HCMECs, combined with ferroptosis inducers or inhibitors to evaluate the H/R injury and ferroptosis in cell function, oxidative stress, and mitochondrial morphology, as well as further explored the molecular mechanism of H/R injury affecting HCMECs through ferroptosis.
Methods
Cell culture, model induction, and cell treatment
Human cardiac microvascular endothelial cells (HCMECs) were obtained from ScienCell Research Laboratories, Inc. and cultured in endothelial cell medium with 5 mmol/L glucose and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37 °C with 5% CO2.
Induction of H/R model
HCMECs were first cultured in glucose-free and serum-free endothelial cell medium and exposed to hypoxia environment at 37 °C (94% N2, 5% CO2, and 1% O2) for 12 h to induce hypoxia. Subsequently, the culture medium was replaced with DMEM medium containing 4.5 g/L glucose and supplemented with 10% FBS (Invitrogen, USA), 100 U/mL penicillin, and 100 U/mL streptomycin (Solarbio Science & Technology Co., Ltd., China), and the cells were cultured under normoxic conditions (95% air and 5% CO2) for 24 h at 37 °C to induce reoxygenation.
To explore the effect of ferroptosis, HCMECs were treated with ferrostatin-1 (1 μM) or erastin (10 μM) for 8 h before the H/R model induction.
Adenovirus-mediated gene transfer into HCMECs
The adenovirus vector with ENPP2 gene overexpression was constructed by the manufacturer (Gene Pharma, China). HCMECs were seeded into 24-well culture dishes, and when they reached 50% confluence, cells were transfected with the ENPP2 overexpression adenovirus (Ad. ENPP2) or negative control adenovirus (Ad. null) at a multiplicity of infection of 100.
Tube formation assay
The wells of the 96-well plate were coated with 50 μL of Matrigel® matrix basement membrane (Corning® 354234) and incubated for 30 min, and then HCMECs were seeded on the gel with an average of 1 × 104/well. After incubation for 12 h, the tube formation of HCMECs was observed by a microscope.
Wound-healing assay
For the wound-healing assay, HCMECs of each group were cultured to 100% confluence, and then a scratch was made using a pipet tip (10 μL). After that, HCMECs were cultured with serum-free endothelial cell medium. The wounds were photographed at 0 and 24 h, and the migration distance of the HUVECs was measured.
Western blot
HCMECs from each treatment group were first lysed with RIPA lysis buffer. Then, the total protein concentration was evaluated with the protein assay kit (Beyotime Biotech, China). Total protein concentration was probed with primary antibodies: HGF (1:3000, ab178395; Abcam), GPX4 (1:5000, ab125066; Abcam), VEGF (1:5000, ab32152; Abcam), ACSL4 (1:10,000, ab155282; Abcam), p-ERK (1:1000, ab201015; Abcam), uPA (1:1000, ab218106; Abcam), ENPP2 (1:500, ab77104; Abcam), and GAPDH (1:5000, ab9485; Abcam), followed by detection with an ECL kit (Thermo Fisher Scientific, USA). Finally, the protein was quantitatively analyzed using chemidoc imaging system (Tanon, China) and ImageJ analysis software.
ROS detection
According to the manufacturer’s optimized instructions, the intracellular levels of ROS in HCMECs from different treatment groups were analyzed by the probe of DCFH-DA, a ROS detection kit (Beyotime, China). The cells were collected and suspended in the diluted DCFH-DA (10 μM) and incubated for 20 min in a 37-°C cell incubator. After fully contacting the probe and the cells by reverse mixing, the cells were washed with serum-free cell culture solution to fully remove DCFH-DA. After collecting the cells, the samples loaded with the probe were detected by flow cytometry.
Transmission electron microscopy (TEM)
In order to observe the damage of mitochondrial morphology in HCMECs, we performed TEM observation on cells in each group. Firstly, HCMECs were prefixed with 2% glutaraldehyde and then fixed with 1% osmium tetroxide. Next, the samples were dehydrated in ethanol containing 3% uranyl acetate and embedded in propylene oxide and epoxy resin overnight. Subsequently, the samples were polymerized into slices (70-nm thick) and stained with lead citrate. Eventually, an H-7650 transmission electron microscope (Hitachi) was used for testing and observation.
Statistical analysis
All the data were analyzed with Prism 8.0 (GraphPad, USA), presented as mean ± SEM. For difference comparisons between groups, unpaired Student’s t test or one-way analysis of variance (ANOVA) was employed. p < 0.05 was indicated statistically significant.
Results
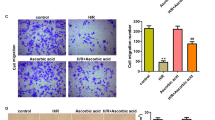
H/R injury inhibited the migration and angiogenesis of HCMECs by exacerbating ferroptosis
To explore the effect of H/R injury and ferroptosis on cellular function of HCMECs, HCMECs were exposed to H/R conditions along with erastin (ferroptosis activator) or ferrostatin-1 (ferroptosis inhibitor). Then, we detected the migration ability and angiogenesis ability of the cells in each group. The wound-healing assay revealed that compared with the control group, the migration ability was apparently restrained in H/R or erastin group, while it was reversed following ferrostatin-1 administration (Fig. 1A, B). As shown in Fig. 1C, tube formation ability of HCMECs was obviously elevated with H/R or/and erastin treatment, while the co-treatment of H/R and ferrostatin-1 induced the induction of angiogenesis (Fig. 1C, D). These results indicated that H/R might inhibit the migration and angiogenesis of HCMECs by regulating ferroptosis.
H/R injury inhibited the migration and angiogenesis of HCMECs by exacerbating ferroptosis. We first established H/R model with HCMECs, with treatment of ferroptosis activator, erastin, or ferroptosis inhibitor, ferrostatin-1, the group of which was named as: control, H/R, H/R erastin, and H/R ferrostatin-1. A, B The wound healing assay was used to detect the migration ability of HCMECs in each group. C, D The tube formation assay was used to detect the angiogenesis ability of HCMECs in each group. Data are presented as the mean ± SEM (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001
H/R injury induced the oxidative stress and mitochondrial damage of HCMECs by exacerbating ferroptosis
Studies have indicated that H/R injury was related to ferroptosis and mitochondrial dysfunction (Chen et al. 2022). Therefore, the next question to address was whether H/R stimulation could regulate oxidative stress and mitochondrial function by exacerbating ferroptosis in HCMECs. We first performed flow cytometry to detect the production of ROS in each group of HCMECs. The results revealed that in the presence of H/R condition or/and erastin treatment, the production of cellular ROS was apparently increased, while it was further decreased under H/R + ferrostatin-1 conditions compared with in H/R group (Fig. 2A, B). In addition, TEM was used to observe the state of mitochondria in each group of cells. There was an increase intracellular swelling, and breakage of mitochondria was observed in H/R condition or/and erastin treatment. Compared with this, there was a significant reduction of mitochondrial swelling and fragmentation in H/R + ferrostatin-1 group (Fig. 2C, D). These observations verified that H/R injury increased ROS production and accelerated mitochondrial damage by exacerbating ferroptosis.
H/R injury and ferroptosis induced the oxidative stress and mitochondrial damage of HCMECs by exacerbating ferroptosis. A, B The production of cellular ROS was analyzed by flow cytometry. C TEM was used to observe the state of mitochondria in each group of cells; the yellow arrows indicate normal mitochondria and blue arrows indicate swollen cracked mitochondria. D Statistical analysis of swollen cracked mitochondria in each group. Data are presented as the mean ± SEM (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001
Mechanisms of H/R injury induced ferroptosis affecting the function of HCMECs
To further confirm the findings of the above results, we detected the function-related proteins of ferroptosis, oxidative stress, and mitochondria in HCMECs. As the results show, the erastin administration could activate oxidative stress characterized by decreasing the protein expression of GPX4, whereas ferrostatin-1 treatment could upregulate GPX4 expression (Fig. 3A, B). H/R condition or/and erastin treatment could induce the ferroptosis by promoting ACSL4, but the expression of ACSL4 was reversed in the H/R + ferrostatin-1 group (Fig. 3A, C). As shown in Fig. 3D, E, the levels of HGF and VEGF were increased under H/R condition or/and erastin treatment, which was then decreased in the H/R + ferrostatin-1 group (Fig. 3A, D, E). In addition, the expression of proteins p-ERK and uPA related to cell proliferation and migration was significantly induced under H/R condition or/and erastin treatment, and both were repressed in the H/R + ferrostatin-1 group, while the expression of ERK did not change significantly (Fig. 3A, F, H). These data verified that the effect of H/R injury on cell function is achieved by exacerbating ferroptosis.
ENPP2 reverses the effect of H/R and ferroptosis on cell migration and angiogenesis in HCMECs
Multiple previous studies have shown that ENPP2 regulates cellular functions, especially cell migration. In order to further explore the molecular mechanism of H/R affecting cell functions by mediating ferroptosis, HCMECs with H/R condition or erastin treatment were transfected with Ad. ENPP2 or Ad. null. The wound-healing test results revealed that compared with the H/R Ad.null or Ad.null erastin group, the cell migration levels in the H/R Ad. ENPP2 and the Ad. ENPP2 erastin groups were significantly increased, indicating that overexpression of ENPP2 can significantly enhance the migration ability of HCMECs (Fig. 4A, B). Similarly, the results of tube formation revealed that in the presence of Ad. ENPP2 transfection group, the tube formation ability of HCMECs was apparently improved compared with the H/R Ad.null or Ad.null erastin group (Fig. 4C, D). The results above suggested that ENPP2 might be involved in the process by which H/R injury inhibits the migration and angiogenesis of HCMECs by regulating ferroptosis.
ENPP2 reverses the effect of H/R and ferroptosis on cell migration and angiogenesis in HCMECs. HCMECs with H/R condition or erastin treatment were transfected with Ad. ENPP2 or Ad. null, and the group of which was named as H/R Ad. null, H/R Ad. ENPP2, Ad. null erastin, and Ad. ENPP2 erastin. A, B The wound healing assay was used to detected the migration ability of HCMECs in each group. C, D The tube formation assay was used to detect the angiogenesis ability of HCMECs in each group. Data are presented as the mean ± SEM (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001
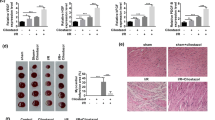
ENPP2 alleviates the effects of H/R and ferroptosis on the oxidative stress and mitochondrial function in HCMECs
Subsequently, we performed flow cytometry and TEM experiments to detect ROS production and observe mitochondrial morphology in each group of HCMECs. The results of flow cytometry showed that the ROS production was markedly depressed in the H/R Ad. ENPP2 or Ad.ENPP2 erastin group compared with that in the H/R Ad.null or Ad.null erastin group (Fig. 5A, B). Furthermore, compared with the Ad.null transfected group, a marked reduction in mitochondrial swelling and fragmentation was observed in the ENPP2 overexpression group (Fig. 5C, D). These results confirmed that overexpression of ENPP2 could bring down the process by which H/R injury increases ROS production and mitochondrial damage by exacerbating ferroptosis.
ENPP2 alleviates the effects of H/R and ferroptosis on the oxidative stress and mitochondrial function in HCMECs. A, B The production of cellular ROS was analyzed by flow cytometry. C TEM was used to observe the state of mitochondria in each group of cells; the yellow arrows indicate normal mitochondria and blue arrows indicate swollen cracked mitochondria. D Statistical analysis of swollen cracked mitochondria in each group. Data are presented as the mean ± SEM (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001
Mechanisms of ENPP2 on hypoxia/reoxygenation (H/R) injury and ferroptosis in HCMECs
The above results confirmed that overexpression of ENPP2 can alleviate the effects of H/R on cell migration, angiogenesis, oxidative stress and mitochondrial damage by regulating ferroptosis. Next, in order to elucidate the molecular mechanism by which ENPP2 is involved in H/R injury in HCMECs, we examined the expression levels of related proteins in HCMECs. As shown in Fig. 6A, the expression of ENPP2 was up-regulated by Ad. ENPP2 in the H/R Ad. ENPP2 or Ad. ENPP2 erastin group compared with that in the H/R Ad.null or Ad.null erastin group (Fig. 6A, B). Overexpression of ENPP2 could obviously induce the protein expression of GPX4, which is a marker protein of oxidative stress (Fig. 6A, C). In contrast to this, overexpression of ENPP2 could obviously repress the protein expression of ACSL4, indicating that the ferroptosis was alleviated in HCMECs (Fig. 6A, D). The angiogenesis-related proteins HGF and VEGF were significantly elevated in the group with the presence of Ad. ENPP2 (Fig. 6A, E, F). Moreover, after overexpression of ENPP2, the cell proliferation- and migration-related proteins p-ERK and uPA were prominently promoted, but ERK expression did not change significantly (Fig. 6A, G, I). Taken together, these findings suggested that the molecular mechanism by which H/R modulates cell migration, angiogenesis, oxidative stress, and mitochondrial damage in HCMECs by affecting ferroptosis may be achieved by regulating ENPP2.
Mechanisms of ENPP2 on hypoxia/reoxygenation (H/R) injury and ferroptosis in HCMECs. A Western blot was used to detect the protein expression of B GPX4, C ENPP2, D ACSL4, E HGF, F VEGF, G p-ERK, H ERK, and I uPA. Data are presented as the mean ± SEM (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001
Discussion
In recent decades, with the improvement of living standards in China, the prevalence of myocardial ischemia has increased year by year, especially in the middle-aged and elderly population (Zhao et al. 2017). Myocardial I/R injury has many hazards such as increasing endothelial permeability, impairing endothelial barrier function, causing cell swelling, microvascular obstruction, and hindering the blood supply of the heart (Gao et al. 2019; Rios-Navarro et al. 2019). Therefore, effective alleviation of I/R injury is imperative for treating of myocardial ischemia.
Previous studies have revealed that the pathophysiological mechanisms underlying I/R injury might participate in some cellular biochemical processes mainly including oxidative stress, apoptosis, ferroptosis, and energy metabolism (He et al. 2022; Kalogeris, Baines, Krenz, and Korthuis 2012), and the processes above are interrelated and may eventually aggravate cell death directly or indirectly (J. Li et al. 2021). As is known to all, a series of injuries caused by oxidative stress is the main reason for the occurrence and development of I/R injury in different organs such as heart, brain, and liver (Xia, Chen, Fan, and Xue 2014; Xia, Chen, Fan, Xue, and Liu 2015). Oxidative stress is characterized by the excessive production of ROS and the activation of excessive lipid peroxidation, which is precisely the cause of ferroptosis, and leads to the damage of mitochondrial respiratory chain and the imbalance of homeostasis (Park et al. 2021; Wu et al. 2018).
Ferroptosis is an example of an iron-mediated mechanism with pathological effects on the heart. More importantly, emerging studies have reported that ferroptosis induces and aggravates tissue damage following organs ischemia such as cerebral (Wang et al. 2022), renal (Friedmann Angeli et al. 2014), and cardiac (H. Liu et al. 2022), whereas the damage could be rescued by ferrostatin-1, substantiating ferroptosis is a strong contributor to I/R injury (Yu, Guo, Xie, Wang, and Chen 2017). Herein, we investigated the effects of H/R injury on HCMECs by mediating ferroptosis, and H/R model was established with HCMECs followed by the treatment of ferroptosis inducer, erastin, or inhibitor ferrostatin-1. Our results showed that ferrostatin-1 could reverse the effect of H/R injury on HCMECs, including the cell migration, angiogenesis, ROS production, and mitochondrial morphology, indicating that H/R injury was achieved through accelerating the process of ferroptosis.
Subsequently, we further explored the molecular mechanism of H/R injury in HCMECs, and ENPP2 attracted our attention. Enpp2 (exonucleotide pyrophosphatase/phosphodiesterase 2; autophagy), a hemolytic phospholipase D from the arterial wall, encourages atherogenic monocyte adhesion by inducing lysophosphatidic acid (LPAS). ENPP2 regulates a variety of biological functions through the homologous G protein-coupled receptor LPAR1-6. Enpp2 promotes tumor cell dispersal, migration, and metastasis through LPAR1 (Auciello et al. 2019; Lin et al. 2019) and T cell motility through LPAR2 (Knowlden et al. 2014; Takeda et al. 2016). Most notably, ENPP2 protects cardiomyocytes from erastin-induced ferroptosis, and ENPP2 overexpression notably promotes migration and proliferation and suppresses erastin-induced ferroptosis of H9c2 cells (Bai et al. 2018). So far, in our study findings, we demonstrated that ENPP2 overexpression had an ability to prevent the development of H/R injury through blocking ferroptosis.
To sum up, our study identified that the injury of myocardial I/R to HCMECs might concern cell migration ability, cellular oxidative stress, ferroptosis level, and mitochondrial function. Moreover, we also emphasize that ENPP2 has been commonly used in the study on tumor progression previously, but our data show that it can also participate in myocardial I/R injury by regulating the level of oxidative stress and ferroptosis, but the specific downstream targets and related signaling pathways need to be further studied. As a supplement to previous studies, our findings establish a theoretical foundation for clinical treatment of myocardial I/R.
References
Ambrose JA (2006) Myocardial ischemia and infarction. J Am Coll Cardiol 47(11 Suppl):D13–D17. https://doi.org/10.1016/j.jacc.2006.04.013
Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, Sherman MH (2019) A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov 9(5):617–627. https://doi.org/10.1158/2159-8290.Cd-18-1212
Bai YT, Chang R, Wang H, Xiao FJ, Ge RL, Wang LS (2018) ENPP2 protects cardiomyocytes from erastin-induced ferroptosis. Biochem Biophys Res Commun 499(1):44–51. https://doi.org/10.1016/j.bbrc.2018.03.113
Binder A, Ali A, Chawla R, Aziz HA, Abbate A, Jovin IS (2015) Myocardial protection from ischemia-reperfusion injury post coronary revascularization. Expert Rev Cardiovasc Ther 13(9):1045–1057. https://doi.org/10.1586/14779072.2015.1070669
Chen GH, Song CC, Pantopoulos K, Wei XL, Zheng H, Luo Z (2022) Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic Biol Med 180:95–107. https://doi.org/10.1016/j.freeradbiomed.2022.01.012
Conrad M, Kagan VE, Bayir H, Pagnussat GC, Head B, Traber MG, Stockwell BR (2018) Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev 32(9-10):602–619. https://doi.org/10.1101/gad.314674.118
Cui H, Li N, Li X, Qi K, Li Q, Jin C, Yang Y (2016) Tongxinluo modulates cytokine secretion by cardiac microvascular endothelial cells in ischemia/reperfusion injury. Am J Transl Res 8(10):4370–4381
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Conrad M (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191. https://doi.org/10.1038/ncb3064
Gao XM, Su Y, Moore S, Han LP, Kiriazis H, Lu Q, Du XJ (2019) Relaxin mitigates microvascular damage and inflammation following cardiac ischemia-reperfusion. Basic Res Cardiol 114(4):30. https://doi.org/10.1007/s00395-019-0739-9
García N, Zazueta C, Aguilera-Aguirre L (2017) Oxidative stress and inflammation in cardiovascular disease. Oxid Med Cell Longev 2017:5853238. https://doi.org/10.1155/2017/5853238
González-Montero J, Brito R, Gajardo AI, Rodrigo R (2018) Myocardial reperfusion injury and oxidative stress: therapeutic opportunities. World J Cardiol 10(9):74–86. https://doi.org/10.4330/wjc.v10.i9.74
He J, Liu D, Zhao L, Zhou D, Rong J, Zhang L, Xia Z (2022) Myocardial ischemia/reperfusion injury: mechanisms of injury and implications for management (Review). Exp Ther Med 23(6):430. https://doi.org/10.3892/etm.2022.11357
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/b978-0-12-394309-5.00006-7
Kandula, V., Kosuru, R., Li, H., Yan, D., Zhu, Q., Lian, Q., . . . Irwin, M. G. (2016). Forkhead box transcription factor 1: role in the pathogenesis of diabetic cardiomyopathy. Cardiovasc Diabetol, 15, 44. https://doi.org/10.1186/s12933-016-0361-1
Knowlden SA, Capece T, Popovic M, Chapman TJ, Rezaee F, Kim M, Georas SN (2014) Regulation of T cell motility in vitro and in vivo by LPA and LPA2. PLoS One 9(7):e101655. https://doi.org/10.1371/journal.pone.0101655
Lejay A, Fang F, John R, Van JA, Barr M, Thaveau F, Scholey JW (2016) Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol 91:11–22. https://doi.org/10.1016/j.yjmcc.2015.12.020
Li H, Xia Z, Chen Y, Qi D, Zheng H (2018) Mechanism and therapies of oxidative stress-mediated cell death in ischemia reperfusion injury. Oxid Med Cell Longev 2018:2910643. https://doi.org/10.1155/2018/2910643
Li W, Li W, Leng Y, Xiong Y, Xia ZJD, biology, c. (2020) Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA and cell biology 39(2):210–225. https://doi.org/10.1089/dna.2019.5097
Li J, Liu S, Yao R, Tian Y, Yao YJF (2021) A novel insight into the fate of cardiomyocytes in ischemia-reperfusion injury: from iron metabolism to ferroptosis. Frontiers in Cell and Developmental Biology 9:799499. https://doi.org/10.3389/fcell.2021.799499
Lin S, Haque A, Raeman R, Guo L, He P, Denning TL, Yun CC (2019) Autotaxin determines colitis severity in mice and is secreted by B cells in the colon. Faseb j 33(3):3623–3635. https://doi.org/10.1096/fj.201801415RR
Liu, Y., Lian, K., Zhang, L., Wang, R., Yi, F., Gao, C., . . . Tao, L. (2014). TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol, 109(5), 415. https://doi.org/10.1007/s00395-014-0415-z
Liu H, Mo H, Yang C, Mei X, Song X, Lu W (2022) A novel function of ATF3 in suppression of ferroptosis in mouse heart suffered ischemia/reperfusion. Free Radical Biology and Medicine 189:122–135. https://doi.org/10.1016/j.freeradbiomed.2022.07.006
Pagliaro BR, Cannata F, Stefanini GG, Bolognese L (2020) Myocardial ischemia and coronary disease in heart failure. Heart Fail Rev 25(1):53–65. https://doi.org/10.1007/s10741-019-09831-z
Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Moon JS (2021) NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol 41:101947. https://doi.org/10.1016/j.redox.2021.101947
Zhao M, Klipstein-Grobusch K, Wang X, Reitsma JB, Zhao D, Grobbee DE, Vaartjes I (2017) Prevalence of cardiovascular medication on secondary prevention after myocardial infarction in China between 1995-2015: a systematic review and meta-analysis. PLoS One 12(4):e0175947. https://doi.org/10.1371/journal.pone.0175947
Rios-Navarro C, Marcos-Garces V, Bayes-Genis A, Husser O, Nuñez J, Bodi V (2019) Microvascular obstruction in ST-segment elevation myocardial infarction: looking back to move forward. Focus on CMR. J Clin Med 8(11):1805–1824. https://doi.org/10.3390/jcm8111805
She X, Lan B, Tian H, Tang BJF, i. n. (2020) Cross talk between ferroptosis and cerebral ischemia. 14:776. https://doi.org/10.3389/fnins.2020.00776
Shi B, Ma M, Zheng Y, Pan Y, Lin X (2019) mTOR and Beclin1: two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J Cell Physiol 234(8):12562–12568. https://doi.org/10.1002/jcp.28125
Shi Y, Han L, Zhang X, Xie L, Pan P, Chen F (2022) Selenium alleviates cerebral ischemia/reperfusion injury by regulating oxidative stress, mitochondrial fusion and ferroptosis. Neurochem Res 47(10):2992–3002. https://doi.org/10.1007/s11064-022-03643-8
Singhal AK, Symons JD, Boudina S, Jaishy B, Shiu YT (2010) Role of endothelial cells in myocardial ischemia-reperfusion injury. Vasc Dis Prev 7:1–14. https://doi.org/10.2174/1874120701007010001
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Chen Y (2018) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23(1):101–113. https://doi.org/10.1007/s12192-017-0827-4
Takeda, A., Kobayashi, D., Aoi, K., Sasaki, N., Sugiura, Y., Igarashi, H., . . . Umemoto, E. (2016). Fibroblastic reticular cell-derived lysophosphatidic acid regulates confined intranodal T-cell motility. Elife, 5, e10561. https://doi.org/10.7554/eLife.10561
Wang P, Ren Q, Shi M, Liu Y, Bai H, Chang YJA (2022) Overexpression of mitochondrial ferritin enhances blood-brain barrier integrity following ischemic stroke in mice by maintaining iron homeostasis in endothelial cells. 11(7). https://doi.org/10.3390/antiox11071257
Wu C, Zhao W, Yu J, Li S, Lin L, Chen X (2018) Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Sci Rep 8(1):574. https://doi.org/10.1038/s41598-017-18935-1
Xia Z, Chen Y, Fan Q, Xue M (2014) Oxidative stress-mediated reperfusion injury: mechanism and therapies. Oxid Med Cell Longev 2014:373081. https://doi.org/10.1155/2014/373081
Xia Z, Chen Y, Fan Q, Xue M, Liu KX (2015) Oxidative stress-mediated reperfusion injury 2014. Oxid Med Cell Longev 2015:689416. https://doi.org/10.1155/2015/689416
Yu H, Guo P, Xie X, Wang Y, Chen G (2017) Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med 21(4):648–657. https://doi.org/10.1111/jcmm.13008
Zhang B, Sun C, Liu Y, Bai F, Tu T, Liu QJO (2022) Exosomal miR-27b-3p derived from hypoxic cardiac microvascular endothelial cells alleviates rat myocardial ischemia/reperfusion injury through inhibiting oxidative stress-induced pyroptosis via FOXO1/GSDMD signaling. Oxidative Medicine and Cellular Longevity 2022:8215842. https://doi.org/10.1155/2022/8215842
Acknowledgements
We would like to thank all the researchers and study participants for their contributions.
Funding
This work was supported by the Natural Science Foundation of Fujian Province (grant number 2020J01122893).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guanhua Fang is the first author. Yanming Shen is the co-first author.
Highlights
• H/R injury inhibited the migration and angiogenesis of HCMECs by exacerbating ferroptosis.
• H/R injury induced the oxidative stress and mitochondrial damage of HCMECs by exacerbating ferroptosis.
• H/R injury induced ferroptosis affecting the function of HCMECs through regulating ENPP2 expression.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, G., Shen, Y. & Liao, D. ENPP2 alleviates hypoxia/reoxygenation injury and ferroptosis by regulating oxidative stress and mitochondrial function in human cardiac microvascular endothelial cells. Cell Stress and Chaperones 28, 253–263 (2023). https://doi.org/10.1007/s12192-023-01324-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-023-01324-1