Abstract
Diabetic cardiomyopathy (DCM) is a disorder of the heart muscle in people with diabetes that can occur independent of hypertension or vascular disease. The underlying mechanism of DCM is incompletely understood. Some transcription factors have been suggested to regulate the gene program intricate in the pathogenesis of diabetes prompted cardiac injury. Forkhead box transcription factor 1 is a pleiotropic transcription factor that plays a pivotal role in a variety of physiological processes. Altered FOXO1 expression and function have been associated with cardiovascular diseases, and the important role of FOXO1 in DCM has begun to attract attention. In this review, we focus on the FOXO1 pathway and its role in various processes that have been related to DCM, such as metabolism, oxidative stress, endothelial dysfunction, inflammation and apoptosis.
Similar content being viewed by others
Background
Diabetic cardiomyopathy (DCM) is causative for 80 % of the fatality rate in the diabetic inhabitants [1, 2]. The molecular theory of DCM describes that, hyperglycaemia is the core pathogenic cause, which roots irregularities at the cardiac myocyte level, ultimately contributing to structural and functional anomalies [2]. This is also nurtured by the datum that patients with diabetes mellitus, independently of the viciousness of coronary artery disease, have amplified threat of heart failure in contrast with subjects deprived of diabetes mellitus [3, 4].
DCM linked pathologies have their ancestries in shifts of gene expression. Although various origins of cardiac injury have been identified, the vital molecular contraptions of their pathogenesis have not been anticipated. The molecular operations within the cardiac cell are organised by transcription factors such as forkhead box (other) transcriptional factor (FOXO). In heart diseases such as atherosclerosis [5], diabetic cardiomyopathy [6, 7], there is an augmented FOXO activity. In this paper we will focus on the possible role of FOXO regulation in DCM. This entity embraces the direct outcome of diabetes mellitus, as one of the utmost rampant diseases, on the myocardium. FOXO regulation may deed in DCM via numerous pathways, by governing different set of genes being involved in allied processes such as oxidative stress, metabolism, inflammation, endothelial dysfunction and apoptosis.
FOXO’s
The “forkhead” name was conferred on account of the two spiked-head structures observed in the embryos of the Drosophila Melanogaster forkhead mutant and its part was connected to the development of gut of the Drosophila fetus [8]. Forkhead proteins are identified as novel class of transcription factors in the late 20th century [9]. FOXO is one amongst 19 families of FOX superfamily and incorporates FOXO1, 3, 4, and 6 [10, 11]. FOXO in humans is similar to dFOXO in Drosophila Melanogaster, and abnormal dauer formation-16 in Caenorhabditis elegans [10].
FOXO’s consists of highly conserved forkhead/winged helix DNA-binding domain, which encompasses the most common 110 amino acids of FOXO family and embodies 3a, 3b, and 2 winged helices, facilitating its DNA binding [12, 13]. FOXO1 and FOXO3 are expressed globally, and FOXO1 isoform is abundantly located in hepatic, fatty tissue and pancreatic β cells [14, 15]. FOXO4 is mainly located in muscle, renal, and colorectal tissue while FOXO6 is predominantly located in the liver and cerebrum [16]. Post-translational modifications (PTM) such as phosphorylation, acetylation, ubiquitination, arginine methylation, and O-glycosylation [13, 17] are known to determine the FOXO1 nuclear transit and transcriptional activity [18]. These modifications can either enhance or reduce the FOXO1 transcriptional activity as determined by the upstream target and/or the sites concerned [17]. AKT phosphorylates FOXO1, facilitating its nuclear transit, which consecutively decreases the transcriptional function of FOXO1 [19–21]. However, several other kinases like mitogen-activated protein kinases (also known as JNKs), cyclin-dependent kinase 2 and nuclear factor κB (NFκB) kinase are also involved in FOXO1 phosphorylation [22–24]. The nuclear compartmentalization and transcriptional function of FOXO1 can also be modified by other PTM like acetylation, ubiquitina-tion, glycosylation and methylation [25–29].
Over the recent decade, numerous studies have uncovered the essential functions of FOXOs in managing diverse range of cellular processes. FOXO1 is the key member among the ‘O’ subfamily, in controlling equilibrium of cardiac cells [18, 30]. Global loss of FOXO1 is fatal as it initiates embryonic cell death because of inadequate vascular growth [31]. From the embryo to adulthood, FOXO factors play an important role in maintaining cardiac homeostasis [32]. Furthermore, FOXO1 is concerned in controlling cellular responses like oxidative stress response, cell multiplication, immune homeostasis, cell death, and metabolism in diverse kinds of tissues [33].
FOXO regulated genes
In relation to the heart, FOXO controls the expression of a variety of target genes that are involved in cellular metabolism, oxidative stress, apoptosis and cell cycle differentiation (Table 1). Interestingly, FOXO factors have been shown to be regulated by numerous stress stimuli, including DNA damage, cytokines, nutrient and oxygen deprivation [24, 34–39]. In addition, stimulation of FOXO factors by 5′ adenosine monophosphate-activated protein kinase stimulates the preferential expression of a gene expression program that heightens cellular stress resistance [37, 38]. In spite of the fact that the regulation of FOXO components is majorly controlled by posttranslational changes, a series of latest studies have emphasized how FOXO factors additionally coordinate extracellular stimuli through substitute mechanisms. For instance, the growth regulatory cytokine such as transforming growth factor β triggers the expression of genes involved in cell cycle inhibition like p15 and p21 through complex formation between FOXO, Smad, and C/EBPb transcription factors at particular promoters [34–36, 40, 41]. These most recent studies highlight the complex regulation of the FOXO transcription factors, by an extensive variety of different stimuli, including cytokines, glucose availability, DNA damage and oxygen deprivation, that may aid to refine FOXO function in distinctive cell kinds under diverse environmental settings.
The role of FOXO1 in the heart
Among the FOXO subfamily, FOXO1, FOXO3, FOXO4 are expressed in the heart [57] and localizes in nucleus where they get associated with coactivators and modulate multiple signal transduction pathways [34, 58–60]. The impacts of FOXO family on heart function and cardiac remodelling have been reviewed [61, 62]. Within the setting of cardiac function, the FOXO proteins are supposed to be participated in oxidative stress [63], regulation of metabolism [58], cell cycle [64], and cell death [65]. Fetal development of heart obliges cell development and division, and FOXO1 is essential for embryonic vascular and cardiac development. For instance, FOXO1 gene knocked out embryos die at E10.5–E11 day and demonstrated curbed vascular and cardiac growth [31, 66]. On the contrary, mice overexpressing a myocardial specific FOXO1 also die by E10.5 because of anomalous expression of cyclin-dependent kinase inhibitors p21 (cip1) and p27 (kip1), resulting in diminished proliferation of cardiomyocytes, decreased cardiac size and myocardium thickness, and ensuing cardiac catastrophe [67]. Myocardial SIRT1 overexpression and precludes aging of heart via FOXO1 mediated induction of catalase expression [51].
FOXO1 and cardiovascular diseases
Dysregulated activity of FOXO1 has been implicated in the pathophysiology of DCM [6, 7, 68], ischemic heart disease [69] and cardiac hypertrophy [54]. In general, FOXO1 has been found to play a protective role in ischemic heart diseases. For instance, Benzhi Cai et al. [70], reported that deletion of FOXO1 in heart caused an increment in myocardial Na+ load by augmenting NaV1.5, a principle α subunit of the cardiac sodium ion channel and Na+ channel subunit β3 mRNA and resulted in shortening of QRS complex significantly, proposing that FOXO1 is facilitating the modulation of sodium channel in ischemic cardiomyopathy. The protective role of FOXO1 is further confirmed by Sengupta et al. who observed that in mice with cardiac-specific deficiency of both FOXO1 and FOXO3 the hearts demonstrated lowered systole, enlarged scar development and amplified apoptosis relative to control mice, when subjected to myocardial infarction through surgical ligation of coronary artery [71]. Moderate increment of FOXO1 expression in heart diminished while its cardiac overexpression in diabetes [72] or deficiency aggravated myocardial ischemia reperfusion injury [71]. The role of FOXO1 in mediating diabetic heart susceptibility to ischemia–reperfusion injury has recently been reviewed by our group [72]. Besides, in light of an ischemia–reperfusion convention, the FOXO1 and FOXO3 dual knockout mice displayed diminished expression of catalase and Manganese superoxide dismutase (MnSOD). Furthermore, both FOXO1 and FOXO3 transcription factors prevent cardiac hypertrophy by stimulating the expression of atrogin-I (an E3 ubiquitin ligase) that facilitates the inhibition of calcineurin/nuclear factor of activated T cells [54]. In addition, ubiquitinization of FOXO1 by atrogin-I promote its nuclear retention and enhancement of its transcriptional activity, and facilitate to oppose the Akt-dependent physiological hypertrophy [54, 73]. In contrast to the protective role, Yajuan Qi et al. [6] recently observed that FOXO1 plays a prominent role in the development of DCM. FOXO1 upregulation in insulin resistance state may lead to impairment of cardiac contractility by increasing β-myosin heavy chain gene expression in cardiac cells [6].
FOXO1 and DCM
Diabetic patients are more prone to the risk of cardiomyopathy, and heart failure is a foremost cause of death in diabetes populations [74–76]. This could not be explicated by considering several other diabetes related risk factors such as dyslipidemia, obesity, infarction, endothelial dysfunction. Thus, diabetes mellitus independent of coronary vascular disease and hypertension can modify the structures as well as functions of the myocardium, a state acknowledged as DCM [77]. In this process, the metabolic derangements in glucose and lipids trigger rigorous cardiac changes that progresses to dysfunction of ventricular diastole and systole [78]. Furthermore, lipid overload in the myocardium leads to contractile dysfunction in animal models and humans by upregulating gene expressions of peroxisome proliferator activated receptor (PPAR)α, myosin heavy chain (MHC)-β, and tumor necrosis factor (TNF)-α [79]. In addition, deficiency of muscle ring-finger protein (MuRF)2, an ubiquitin ligase resulted in the development of diabetic cardiomyopathy by enhancing cardiac PPARα and PPARγ1 gene expression [80]. However, cellular mechanisms associated with DCM, including FOXO1 signalling, are not yet completely understood [77]. Understanding for the pathogenesis of DCM is stemmed mainly from in vivo animal models [81, 82]. Recent in vivo and in vitro studies indicate that enhanced cardiac FOXO1 activation has been illustrated in diabetic mice [7]. The responses in the heart such as metabolic adaptation, oxidative stress, endothelial dysfunction, inflammation, and apoptosis in which FOXO1 could participate that may lead to DCM are illustrated below (Fig. 1).
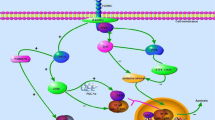
Regulation and function of FOXO-1 in the development of DCM. In diabetes mellitus, various stimuli like excess glucose, excess lipids, oxygen free radicals, cytokines and other growth factors triggers several mechanisms that promote posttranslational modifications like phosphorylation, acetylation, deacetylation which may regulate the FOXO-1 activity and function. Akt promotes the phosphorylation and translocation of FOXO1 to cytosol and facilitates its binding with 14-3-3 protein which directs it for degradation whereas protein phosphatase 2A (PP2A) causes dephosphorylation and translocates FOXO1 to nucleus from cytosol. E3 ubiquitin ligase facilitates ubiquination of FOXO1, while ubiquitin specific protease (USP7) inverted the process. Further, phosphorylation by Mst1 stimulates FOXO1 transcriptional activity. In addition, cAMP response element binding protein (CBP) and p300 histone acetyltransferase acetylates FOXO1, and silent information regulator 1 (SIRT1) deacetylates it. Activated FOXO-1 binds to the FOXO-binding site and triggers several genes involved in inflammation, oxidative stress, nitrosative stress, glucose and lipid metabolism, hypertrophy, autophagy and apoptosis that finally leads to alteration of cardiac structure, metabolism, function and cardiac cell death. P phoshorylation; Ub ubiquitination; Ac acetylation
FOXO1 and DCM-associated metabolism
Disturbances in myocardial glucose and lipid metabolism are initial events that lead to cardiac dysfunction in diabetic condition. FOXO1 is involved in various pathways related to cellular energy metabolism. During insulin resistance, pyruvate dehydrogenase kinase 4 (PDK4) is known to inhibit glucose oxidation by blocking pyruvate to enter into mitochondrial oxidation through phosphorylating the E1 moiety of pyruvate dehydrogenase complex [83]. Enhanced expression of FOXO1 downstream target gene PDK4 gene is also observed in high fat and obese animal models of insulin resistance, which adversely regulate insulin actions [84, 85]. FOXO1 can modulate glucose metabolism in adult cardiomyocytes in insulin resistance and diabetic conditions by inhibiting glucose oxidation preceded by PDK4 activation, subsequently altering the substrate preference for fatty acid and lactate [33, 83]. In addition, FOXO1 upholds gluconeogenesis in liver by enhancing G6Pase and PEPCK mRNA [86, 87]. Besides, FOXO1 depletion abolished the high-fat diet induced aberration of glycolytic genes (declined hexokinase 1 and glucose transporter 4) and lipid oxidation gene expression patterns (diminished PGC-1α and amplified PDK4). These findings collectively suggest that FOXO1 is a key factor responsible for anomaly of glucose and lipid metabolic pathways in insulin resistance.
Surprisingly, Battiprolu and his colleagues noticed morphological and functional myocardial modifications similar to DCM in two dissimilar animal models of type 2 diabetes, db/db mice lacking leptin receptor and high-fat diet obese mice, as an outcome of FOXO boosted pathological alterations. Moreover, mice with cardiac specific FOXO1 knock out were resistant to high-fat diet provoked myocardial dysfunction and hypertrophy [7]. Recent evidences suggest that FOXO1 plays a key role in regulating cardiac aberrant metabolisms (i.e.; glucose and fatty acid metabolism) leading to DCM [7, 83]. These studies concludes that FOXO1 stimulation promote cardiac remodelling by altering cardiac metabolism whereas FOXO1 deletion resists cardiac remodelling. Thus, FOXO1 was distinguished as a central player in the cardiac metabolic abnormalities in DCM.
Another cardinal feature of DCM is lipotoxicity resulting in part from excessive lipid accumulation [79]. Puthanveetil et al. suggested that FOXO–iNOS–cluster domain 36 transporter (CD36) axis was involved in lipid accumulation in cardiomyocytes under lipid excess conditions. Oversupply of lipids augments the nuclear localization of FOXO1 accompanied by augmented CD36 translocation to the membrane without affecting its mRNA or total protein content, ensued by amplified lipid oxidation and triglyceride accrual [30]. Further, FOXO1 nuclear compartmentalization and enhancement of its transcriptional activity was observed especially during diabetes and obesity [88, 89]. Enhanced fatty acid flux into the cardiac cell can boost its oxidation with subsequent production of reactive oxygen/nitrogen species [90], which can subsequently induce hypertrophy and cardiac failure [91]. Additionally, FOXO1 has indispensable role in positive regulation of adipocyte fatty acid binding protein (FABP4) gene transcription, thereby controlling uptake and accumulation of lipids in macrophages, and promoting atherosclerosis [92]. Thus, FOXO1 over-activation plays a prominent role in myocardial lipid accumulation as a result of augmented lipid uptake over deployment.
Latest studies have disclosed the FOXO1 ability to relate insulin pathway to numerous kinds of metabolic stress in the heart. FOXO1 indirectly controls the insulin sensitivity via negatively modulating the insulin sensing genes. Insulin sensitivity is restored upon FOXO1 restricted deletion in genetic mouse model of insulin resistance, and this conditional deletion further reduced the expression of genes related to gluconeogenesis (e.g., G6pase and PEPCK1) in the liver and enhanced insulin sensitizing genes expression (e.g., Leptin gene, PPARγ, and solute carrier family two (facilitated glucose transporter), member four) in adipocytes, thereby salvaged the diabetic phenotype [93]. Battiprolu et al. further demonstrated that FOXO mediated feedback control of insulin signalling played an essential role in DCM through inactivation of insulin receptor substrate 1 (IRS-1) [7]. In a preceding study, Niet et al. reported that FOXO stimulation promoted insulin resistance and impairment of glucose metabolism in primary cardiomyocytes through modulating Akt phosphorylation [94]. These observations of the FOXO-regulated vicious cycle of insulin resistance offer novel perceptions in the understanding of the pathogenesis of DCM and other diabetic complications.
The major role of FOXO factors in myocardial metabolic stress adaptation may be correlated through their regulation of autophagy [95, 96], a characteristic of numerous versatile responses of the cardiomyocyte, comprising the reactions to starvation, ischemia–reperfusion, and pressure overload [97, 98]. FOXO1 deacetylation by sirt1 (a class III histone deacetylase) seems to be an essential module of the autophagic reaction to nutrient deficit, and possibly different types of metabolic stress in cardiomyocytes [99]. In the heart, FOXO transcription factor can promote autophagy by stimulating autophagic genes such as Atg12 and Gabarapl1 under stress circumstances [100].
FOXO1 and DCM-linked oxidative stress
Hyperglycemia and the subsequent formation of advanced glycation end-products are major culprits for the generation of ROS within the cardiac tissue in diabetes [101]. In addition, excess mitochondrial oxidation resulting primarily from lipid degradation is another cause for oxidative stress in cardiomyocytes [102]. ROS like hydroxyl, superoxide anion and H2O2 are extremely irritable and can trigger destruction to lipids, proteins and DNA [103]. As oxidative stress plays a significant role in the development of DCM [104], antioxidant therapy can be beneficial for the amelioration of DCM. Recently, rutin, a flavonoid antioxidant was shown to attenuate myocardial ventricular dysfunction and cardiac remodeling in diabetic condition [105]. Hyperglycemia provoked disruption of cellular protective antioxidant mechanisms has been associated in the progression of cardiovascular ailments. Xiaonan Li et al. reported that FOXO1 takes part in the mediation of high glucose induced elevation of oxidative stress that prompted the dysregulation of thioredoxin (Trx) antioxidant system [47]. Hyperglycemia promotes coupling of FOXO1 to the Txnip promoter that is facilitated by p38 MAPK pathway [47]. FOXO1 seems to stimulate cell demise, particularly in tissues that are influenced by diabetes associated complications where oxidative stress is beyond normal limits [106]. However, FOXO1 has an imperative role in cellular protection against oxidative stress by inducing enzymes such as MnSOD and catalase that catalyse ROS [63, 107]. FOXO1 can safeguard pancreatic β-cells against oxidative stress [108].
FOXO1 and DCM-related endothelial dysfunction
Endothelial dysfunction is the harbinger of atherosclerosis that drives the detrimental consequence of diabetes on the heart. Endothelial dysfunction has been revealed in both type 1 and type 2 diabetic patients [109, 110] as well as in several animal models of diabetes [111]. The plausible mechanisms by which FOXO1 causes some features of endothelial dysfunction include the controlling of the expression of both isoforms of nitric oxide synthase enzyme i.e. inducible and endothelial isoforms. It is apparent that impaired endothelial nitric oxide synthase (eNOS) activity leads to endothelial dysfunction [112]. FOXO1 repress transcription of eNOS in endothelial cells [113]. In consistent with this, a recent study by Lee et al. showed that malfunction of FOXO1/KLF2/eNOS signalling promotes diabetic endothelial dysfunction [48]. Besides eNOS regulation, gain of function of FOXO1 in vascular endothelial cells further increased iNOS mRNA with resultant endothelial dysfunction [5]. This is in consistent with the finding that FOXO1 deletion in streptozotocin induced diabetic mice attenuated lipid peroxides and aortic iNOS activation in vascular endothelial cells.
Hyperglycemia-induced oxidative stress promotes post translational modifications of FOXO1 and its nuclear translocation, accounting for its enhanced transcriptional activity, thereby its participation in the progression of atherosclerosis in diabetic patients [5, 49]. Li et al., employed FOXO1 KR/KR knock-in mice to mimic the effect of oxidative stress- (or hyperglycemia-) induced FOXO1 deacetylation on atherosclerosis and demonstrated that FOXO1 gain of function in vascular endothelial cells trump its beneficial effects to lower triglycerides and low density lipoprotein cholesterol levels in its counterpart WTD-fed Ldlr-/- mice, suggesting that FOXO1 is involved in primary atherogenic abnormality occurred in the vascular endothelium [114]. Furthermore, unbridled activity of FOXO1, as a result of hyperglycemia induced O-glycosylation, accounted for its disproportionate regulation of apoptotic and proapoptotic factors (decreased Bcl-2 and increased caspase-3 and BAD), thereby contributing to endothelial cell death in human aorta endothelial cells [49]. In addition to this, any interruption in the endothelin (ET)1-Akt-FOXO1 feedback loop may be a contributing element for ET-1 deregulation and endothelial dysfunction in inadequately managed diabetes mellitus [49].
FOXO1 and DCM-induced inflammation
Inflammation has deemed as a prime causative factor in diabetes and linked with the occurrence of heart failure in DCM. It has been reported that diverse stimuli like high glucose, TNF-α and lipopolysaccharides regulate the expression of proinflammatory cytokines via FOXO1 [106]. In insulin resistant obese mice model, macrophages have amplified FOXO1 stimulation with concomitant elevation of IL-1β production. Furthermore, FOXO1 accomplishes IL-1β expression by combining directly with the IL-1βpromoter [115]. On the other hand, inflammatory cytokines stimulate FOXO1 and may be indulged in positive feedback loop. This was supported by the findings of Behl et al. who observed that augmented TNF-α in diabetes stimulated FOXO1 and this in turn further stimulated the expression of TNF-α levels in microvascular endothelial cells [88]. Elevated glucose levels in diabetes also activate toll-like receptor (TLR) pathway, which causes long-lasting inflammation and tissue injury. A recent study disclosed that FOXO1 supports inflammation during diabetes by increasing the expression of TLR4, recommending that FOXO1 may function as an essential regulator of inflammatory reactions during obesity and diabetes mellitus [116]. Since insulin is involved in FOXO1 repression, FOXO1 is stimulated in insulin resistance condition where diminution of insulin signalling pathway prevails, leading to accelerated inflammatory response. Furthermore, cell fate i.e. whether a cell experiences survival or apoptosis is governed by the relative balance between NF-κB and FOXO1, under inflammatory settings where both factors are triggered [117, 118]. This emphasizes the role of FOXO1 transcription factor as a central mediator of inflammation in the perspective of insulin resistance and obesity.
FOXO1 and DCM-involved apoptosis
Cellular damage that progresses to apoptosis can be considered as an important contributing factor to pathology in maladies like diabetes and cardiovascular injury [119]. FOXO1 is the significant contributor in regulating cell death [33]. FOXO1 emerges as a potential regulator of different kinds of cell death during insulin resistance and diabetes. In diabetes, both intrinsic (mitochondrial cytochrome c mediated) and extrinsic (death receptors like Fas or TNF α mediated) apoptosis are reported to be augmented and FOXO1 is suggested to increase the expression of caspases and cell death receptors [120, 121]. Moreover, FOXO1 signalling can increase the proapoptotic gene expression like Bim and Puma [122, 123] and BAD [30]. Puthanveetil et al. suggested that FOXO1 regulates BAD, a pro-apoptotic factor through PP2A induction in in vivo models of diabetes and insulin resistance [30]. However, FOXO1 overexpression in cardiomyocytes suppresses the PP2A/B activity [94]. The discrepancy between the in vivo and in vitro models in relation to the effect of FOXO1 on PP2A could be the presence of excess lipids brought in by the FOXO1–CD36 pathway observed with diabetes, which turns on PP2A, thereby activating BAD stimulated apoptotic process.
FOXO1 and DCM-associated mitochondrial dysfunction and calcium handling
Mitochondrial malfunction associated with insulin resistance is a prime contributing factor for DCM. FOXO1 is involved in the integration of mitochondrial function with insulin signalling. Elevated FOXO1 levels in insulin resistant states disrupt mitochondrial electron transport chain, thereby promoting impaired oxidative respiration [124]. In addition, FOXO1 is involved in the regulation of the mitochondrial biogenesis by affecting the expression of genes regulating mitochondrial fission and fusion through SIRT1/PGC1α pathway [124].
Stringent regulation of intracellular calcium homeostasis is essential for normal maintenance of cardiac function and growth [125]. Numerous challenges like accumulation of long-chain acetylcarnitines and oxidative stress can lead to impairment of calcium homeostasis in DCM [126]. DCM has been associated with altered function of sarco-endoplasmic reticulum Ca2+-ATPase, and Na+/Ca2+ exchanger (NCX) [127–129]. Altered calcium handling contributes to endoplasmic reticulum stress [130] and is the underlying cause for FOXO1 aggravation of diabetic cardiomyocyte cell death in response to ischemic insult [131]. In addition, enhanced levels of interleukin (IL)-1β contributed to endoplasmic reticulum stress induced DCM via IL-1 receptor-associated kinase-2/C/EBP homologous protein pathway [132]. It has been identified that calcium calmodulin-dependent kinase II has profound effect on FOXO1 nuclear retention, which may lead to excessive glucose production in the liver, in the context of obesity [133]. Surprisingly, in failing cardiomyocytes, enhanced cytoplasmic calcium levels helps facilitate calcium/calmodulin-dependent protein kinase dependent stimulation of Akt which leads to down regulation of microRNA-1 and NCX-1 expression by inhibiting FOXO3A activity [134]. Altogether, these studies implicate the role of FOXO1 in DCM associated mitochondrial dysfunction and calcium handling.
Conclusions
In summary, FOXO1 regulation may contribute to the detrimental outcomes of the cardiac cells in diabetes, accelerating the development of DCM, one of the predominant cardiac difficulties in diabetic patients. Metabolic alterations, oxidative stress, endothelial dysfunction, inflammation and apoptosis have been shown to be implicated in the development and progression of DCM, and also in the desirable processes for the regulation of FOXO1 gene. Dysregulated FOXO1 expression and activity appear to promote endothelial dysfunction, myocardial oxidative stress, cardiomyocyte cell death and inflammation observed in DCM. Thus, FOXO1 or, favourably, any of its distinctive pathways may be of extreme concern for pharmaceutical target. However, mechanisms controlling the activity and expression of FOXO1 isoform in DCM are not well appreciated. There are so many unanswered questions relating to the FOXO1 activity in DCM. Some of them like, what could be the mechanistic link whereby FOXO1 activation contributes to the increased vulnerability of diabetic heart to ischemic insults? What could be the cause for persistent activation of FOXO1 in cardiac tissue in the settings of insulin resistance, lipid overload, elevated inflammatory cytokines and hyperglycemia? Whether FOXO1 plays similar roles like insulin resistance, in non-obese type 1 diabetic patients given that insulin deficiency is another characteristic feature of diabetes? Could FOXO1 gene polymorphism be responsible for individual susceptibility of DCM? What could be the direct metabolic consequences of FOXO1 activation? What is the functional role of FOXO1 in nonmyocytes of the heart? Thus, further research is necessary to unveil the precise mechanism of FOXO1 in the development and progression of DCM.
Abbreviations
- DCM:
-
diabetic cardiomyopathy
- FOXO:
-
forkhead box (other) transcriptional factor
- PTM:
-
posttranslational modifications
- NFκB:
-
nuclear factor κB
- PEPCK:
-
phosphoenolpyruvate carboxykinase
- G6Pase:
-
glucose-6-phosphatase
- apoC-III:
-
apolipoprotein C-III
- PPAR:
-
peroxisome proliferator activated receptor
- PGC-1α:
-
peroxisome proliferator activated receptor γ coactivator 1-α
- PDK4:
-
pyruvate dehydrogenase kinase 4
- Txnip:
-
thioredoxin interacting protein
- iNOS:
-
inducible nitric oxide synthase
- NO:
-
nitric oxide
- KLF2:
-
kruppel-like factor 2
- BAD:
-
B cell leukemia/lymphoma 2-associated death promoter
- MnSOD:
-
manganese superoxide dismutase
- BMP2:
-
bone morphogenic protein 2
- VCAM-1:
-
vascular cell adhesion molecule
- MMP10:
-
matrix metalloproteinase-10
- VEGF:
-
vascular endothelial growth factor
- ROS:
-
reactive oxygen species
- SIRT1:
-
sirtuin1
- H2O2:
-
hydrogen peroxide
- TNF-α:
-
tumor necrosis factor- α
- C/EBPβ:
-
CCAAT/enhancer binding protein
- MCIP1.4:
-
modulatory calcineurin interacting protein exon 4 isoform
- PARP-1:
-
poly(ADP-Ribose)polymerase-1
- ET-1:
-
endothelin-1
- CD36:
-
cluster domain 36 transproter
- A-FABP/FABP4:
-
adipocyte fatty acid binding protein
- IRS-1/2:
-
insulin receptor substrate 1/2
- Trx:
-
thioredoxin
- HO-1:
-
heme oxygenase-1
- eNOS:
-
endothelium nitric oxide synthase
- IL:
-
interlukin
- TLR:
-
toll-like receptor
- PP2A:
-
protein phosphatase 2A
- PDK1:
-
3-phosphoinositide dependent protein kinase-1
- PI3K:
-
phosphatidylinositol-3-kinase
- TGFβ:
-
transforming growth factor beta
- MST1:
-
mammalian homolog of Ser/Thr kinase 1
- USP7:
-
ubiquitin specific protease 7
- CBP:
-
cAMP response element binding protein
- PUMA:
-
p53 upregulated modulator of apoptosis
- GK:
-
glucokinase
- MuRF:
-
muscle ring-finger protein
- ET-1:
-
endothelin-1
- MHC:
-
myosin heavy chain
References
Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85.
Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci. 2004;107(6):539–57.
Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34.
Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–8.
Tanaka J, Qiang L, Banks AS, Welch CL, Matsumoto M, Kitamura T, Ido-Kitamura Y, DePinho RA, Accili D. FOXO1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes. 2009;58(10):2344–54.
Qi Y, Zhu Q, Zhang K, Thomas C, Wu Y, Kumar R, Baker KM, Xu Z, Chen S, Guo S. Activation of FOXO1 by insulin resistance promotes cardiac dysfunction and beta-myosin heavy chain gene expression. Circ Heart Fail. 2015;8(1):198–208.
Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, et al. Metabolic stress-induced activation of FOXO1 triggers diabetic cardiomyopathy in mice. J Clin Investig. 2012;122(3):1109–18.
Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57(4):645–58.
Weigel D, Jackle H. The fork head domain: a novel DNA binding motif of eukaryotic transcription factors? Cell. 1990;63(3):455–6.
Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10(4):233–40.
Daitoku H, Sakamaki J, Fukamizu A. Regulation of FOXO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta. 2011;1813(11):1954–60.
Obsil T, Obsilova V. Structural basis for DNA recognition by FOXO proteins. Biochim Biophys Acta. 2011;1813(11):1946–53.
Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27(16):2263–75.
Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH 3rd, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor FOXO1 links insulin signaling to PDX1 regulation of pancreatic beta cell growth. J Clin Investig. 2002;110(12):1839–47.
Nakae J, Kitamura T, Kitamura Y, Biggs WH 3rd, Arden KC, Accili D. The forkhead transcription factor FOXO1 regulates adipocyte differentiation. Dev Cell. 2003;4(1):119–29.
van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal. 2011;14(4):579–92.
Tzivion G, Dobson M, Ramakrishnan G. FOXO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813(11):1938–45.
Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: developing therapeutic inroads with FOXO proteins. Oxid Med Cell Longev. 2009;2(3):119–29.
Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three AKTS. Genes Dev. 1999;13(22):2905–27.
Kops GJ, Burgering BM. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med (Berl). 1999;77(9):656–65.
Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274(23):15982–5.
Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of FOXO1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57(4):846–59.
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–37.
Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314(5797):294–7.
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–5.
Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates FOXO1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA. 2004;101(27):10042–7.
Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian sirt1 represses forkhead transcription factors. Cell. 2004;116(4):551–63.
Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FOXO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2(3):153–63.
Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by AKT. Mol Cell. 2008;32(2):221–31.
Puthanveetil P, Wang Y, Zhang D, Wang F, Kim MS, Innis S, Pulinilkunnil T, Abrahani A, Rodrigues B. Cardiac triglyceride accumulation following acute lipid excess occurs through activation of a FOXO1-iNOS-CD36 pathway. Free Radic Biol Med. 2011;51(2):352–63.
Hosaka T, Biggs WH, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101(9):2975–80.
Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, Epstein JA, Morrisey EE, Margulies KB, Cappola TP. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114(12):1269–76.
Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Oncogene. 2008;27(16):2320–36.
Seoane J, Le H-V, Shen L, Anderson SA, Massagué J. Integration of smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117(2):211–23.
Gomis RR, Alarcon C, Nadal C, Van Poznak C, Massague J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell. 2006;10(3):203–14.
Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J. A FOXO-smad synexpression group in human keratinocytes. Proc Natl Acad Sci USA. 2006;103(34):12747–52.
Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17(19):1646–56.
Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282(41):30107–19.
Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28(6):941–53.
Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17(19):1635–45.
Siegenthaler JA, Miller MW. Generation of Cajal-Retzius neurons in mouse forebrain is regulated by transforming growth factor beta-Fox signaling pathways. Dev Biol. 2008;313(1):35–46.
Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem. 2000;275(46):36324–33.
Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O’Brien R, Granner DK. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/forkhead proteins. J Biol Chem. 2000;275(39):30169–75.
Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. FOXO1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Investig. 2004;114(10):1493–503.
Ma K, Zhang Y, Elam MB, Cook GA, Park EA. Cloning of the rat pyruvate dehydrogenase kinase 4 gene promoter: activation of pyruvate dehydrogenase kinase 4 by the peroxisome proliferator-activated receptor gamma coactivator. J Biol Chem. 2005;280(33):29525–32.
Qu S, Su D, Altomonte J, Kamagate A, He J, Perdomo G, Tse T, Jiang Y, Dong HH. PPAR{alpha} mediates the hypolipidemic action of fibrates by antagonizing FOXO1. Am J Physiol Endocrinol Metab. 2007;292(2):E421–34.
Li X, Rong Y, Zhang M, Wang XL, LeMaire SA, Coselli JS, Zhang Y, Shen YH. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun. 2009;381(4):660–5.
Lee HY, Youn SW, Cho HJ, Kwon YW, Lee SW, Kim SJ, Park YB, Oh BH, Kim HS. FOXO1 impairs whereas statin protects endothelial function in diabetes through reciprocal regulation of Kruppel-like factor 2. Cardiovasc Res. 2013;97(1):143–52.
Cifarelli V, Lee S, Kim DH, Zhang T, Kamagate A, Slusher S, Bertera S, Luppi P, Trucco M, Dong HH. FOXO1 mediates the autocrine effect of endothelin-1 on endothelial cell survival. Mol Endocrinol. 2012;26(7):1213–24.
Abid MR, Shih SC, Otu HH, Spokes KC, Okada Y, Curiel DT, Minami T, Aird WC. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J Biol Chem. 2006;281(46):35544–53.
Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–21.
Wang YY, Chen SM, Li H. Hydrogen peroxide stress stimulates phosphorylation of FOXO1 in rat aortic endothelial cells. Acta Pharmacol Sin. 2010;31(2):160–4.
Ito Y, Daitoku H, Fukamizu A. FOXO1 increases pro-inflammatory gene expression by inducing C/EBPbeta in TNF-alpha-treated adipocytes. Biochem Biophys Res Commun. 2009;378(2):290–5.
Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, et al. FOXO transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114(11):1159–68.
Sakamaki J, Daitoku H, Yoshimochi K, Miwa M, Fukamizu A. Regulation of FOXO1-mediated transcription and cell proliferation by PARP-1. Biochem Biophys Res Commun. 2009;382(3):497–502.
Wang B, Yang Q, Sun YY, Xing YF, Wang YB, Lu XT, Bai WW, Liu XQ, Zhao YX. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J Cell Mol Med. 2014;18(8):1599–611.
Morris JB, Kenney B, Huynh H, Woodcock EA. Regulation of the proapoptotic factor FOXO1 (FKHR) in cardiomyocytes by growth factors and alpha1-adrenergic agonists. Endocrinology. 2005;146(10):4370–6.
Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–5.
Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, DePinho RA, Kitajewski J, Accili D. A FOXO/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Investig. 2007;117(9):2477–85.
Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308(5725):1181–4.
Ronnebaum SM, Patterson C. The FOXO family in cardiac function and dysfunction. Annu Rev Physiol. 2010;72:81–94.
Ferdous A, Battiprolu PK, Ni YG, Rothermel BA, Hill JA. FOXO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res. 2010;3(4):355–64.
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419(6904):316–21.
Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404(6779):782–7.
Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FOXO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168(10):5024–31.
Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K, et al. Abnormal angiogenesis in FOXO1 (Fkhr)-deficient mice. J Biol Chem. 2004;279(33):34741–9.
Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102(6):686–94.
Relling DP, Esberg LB, Fang CX, Johnson WT, Murphy EJ, Carlson EC, Saari JT, Ren J. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of FOXO3A transcription factor independent of lipotoxicity and apoptosis. J Hypertens. 2006;24(3):549–61.
Dabek J, Owczarek A, Gasior Z, Ulczok R, Skowerski M, Kulach A, Mazurek U, Bochenek A. Oligonucleotide microarray analysis of genes regulating apoptosis in chronically ischemic and postinfarction myocardium. Biochem Genet. 2008;46(5–6):241–7.
Cai B, Wang N, Mao W, You T, Lu Y, Li X, Ye B, Li F, Xu H. Deletion of FOXO1 leads to shortening of QRS by increasing Na(+) channel activity through enhanced expression of both cardiac NaV1.5 and beta3 subunit. J Mol Cell Cardiol. 2014;74:297–306.
Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FOXO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286(9):7468–78.
Li H, Micheael GI, Mingzhe T, Yalan L, Liangqing Z, Xia Z. Role of forkhead transcription factors in myocardial ischemic reperfusion injury in diabetes. J Diabetes Metab. 2013;4:247.
Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008;295(3):H1206–15.
Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26(8):2433–41.
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879–84.
Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126(4):296–306.
Khavandi K, Khavandi A, Asghar O, Greenstein A, Withers S, Heagerty AM, Malik RA. Diabetic cardiomyopathy—a distinct disease? Best Pract Res Clin Endocrinol Metab. 2009;23(3):347–60.
Marwick TH. Diabetic heart disease. Heart. 2006;92(3):296–300.
Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18(14):1692–700.
He J, Quintana MT, Sullivan J, L Parry T, J Grevengoed T, Schisler JC, Hill JA, Yates CC, Mapanga RF, Essop MF, et al. MuRF2 regulates PPARgamma1 activity to protect against diabetic cardiomyopathy and enhance weight gain induced by a high fat diet. Cardiovasc Diabetol. 2015;14:97.
Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115(25):3213–23.
Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98(5):596–605.
Puthanveetil P, Wang Y, Wang F, Kim MS, Abrahani A, Rodrigues B. The increase in cardiac pyruvate dehydrogenase kinase-4 after short-term dexamethasone is controlled by an Akt-p38-forkhead box other factor-1 signaling axis. Endocrinology. 2010;151(5):2306–18.
Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol Genet Metab. 1998;65(2):181–6.
Constantin-Teodosiu D, Constantin D, Stephens F, Laithwaite D, Greenhaff PL. The role of FOXO and PPAR transcription factors in diet-mediated inhibition of PDC activation and carbohydrate oxidation during exercise in humans and the role of pharmacological activation of PDC in overriding these changes. Diabetes. 2012;61(5):1017–24.
Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-glycosylation of FoxO1 increases its transcriptional activity towards the glucose 6-phosphatase gene. FEBS Lett. 2008;582(5):829–34.
Park JM, Kim TH, Bae JS, Kim MY, Kim KS, Ahn YH. Role of resveratrol in FOXO1-mediated gluconeogenic gene expression in the liver. Biochem Biophys Res Commun. 2010;403(3–4):329–34.
Behl Y, Krothapalli P, Desta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58(4):917–25.
Kim JJ, Li P, Huntley J, Chang JP, Arden KC, Olefsky JM. FOXO1 haploinsufficiency protects against high-fat diet-induced insulin resistance with enhanced peroxisome proliferator-activated receptor gamma activation in adipose tissue. Diabetes. 2009;58(6):1275–82.
Lekli I, Mukherjee S, Ray D, Gurusamy N, Kim YH, Tosaki A, Engelman RM, Ho YS, Das DK. Functional recovery of diabetic mouse hearts by glutaredoxin-1 gene therapy: role of Akt-FOXO-signaling network. Gene Ther. 2010;17(4):478–85.
Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93(8):903–7.
Harjes U, Bridges E, McIntyre A, Fielding BA, Harris AL. Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J Biol Chem. 2014;289(33):23168–76.
Nakae J, Biggs WH 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor FOXO1. Nat Genet. 2002;32(2):245–53.
Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FOXO transcription factors activate AKT and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA. 2007;104(51):20517–22.
Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, Lee HC, Lee BW. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11(1):46–59.
Maiese K. FOXO transcription factors and regenerative pathways in diabetes mellitus. Curr Neurovasc Res. 2015;12(4):404–13.
Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–6.
Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16(1):31–8.
Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FOXO by sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107(12):1470–82.
Sengupta A, Molkentin JD, Yutzey KE. FOXO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284(41):28319–31.
Younce CW, Wang K, Kolattukudy PE. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res. 2010;87(4):665–74.
Wang J, Song Y, Wang Q, Kralik PM, Epstein PN. Causes and characteristics of diabetic cardiomyopathy. Rev Diabet Stud. 2006;3(3):108–17.
Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14(4):593–605.
Rosa CM, Xavier NP, Henrique Campos D, Fernandes AA, Cezar MD, Martinez PF, Cicogna AC, Gimenes C, Gimenes R, Okoshi MP, et al. Diabetes mellitus activates fetal gene program and intensifies cardiac remodeling and oxidative stress in aged spontaneously hypertensive rats. Cardiovasc Diabetol. 2013;12:152.
Guimaraes JFC, Muzio BP, Rosa CM, Nascimento AF, Sugizaki MM, Fernandes AAH, Cicogna AC, Padovani CR, Okoshi MP, Okoshi K. Rutin administration attenuates myocardial dysfunction in diabetic rats. Cardiovasc Diabetol. 2015;14:90.
Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;2012:7.
Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295(5564):2450–2.
Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FOXO1. Diabetes Obes Metab. 2007;9(Suppl 2):140–6.
Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88(6):2510–6.
Balletshofer BM, Rittig K, Enderle MD, Volk A, Maerker E, Jacob S, Matthaei S, Rett K, Häring HU. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101(15):1780–4.
De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963–74.
Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88(2):E14–22.
Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of FOXO transcription factors in angiogenesis and postnatal neovascularization. J Clin Investig. 2005;115(9):2382–92.
Qiang L, Tsuchiya K, Kim-Muller JY, Lin HV, Welch C, Accili D. Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of FOXO1 gene. J Biol Chem. 2012;287(17):13944–51.
Su D, Coudriet GM, Hyun Kim D, Lu Y, Perdomo G, Qu S, Slusher S, Tse HM, Piganelli J, Giannoukakis N, et al. FOXO1 links insulin resistance to proinflammatory cytokine IL-1beta production in macrophages. Diabetes. 2009;58(11):2624–33.
Fan W, Morinaga H, Kim JJ, Bae E, Spann NJ, Heinz S, Glass CK, Olefsky JM. FOXO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29(24):4223–36.
Alikhani M, Alikhani Z, Graves DT. FOXO1 functions as a master switch that regulates gene expression necessary for tumor necrosis factor-induced fibroblast apoptosis. J Biol Chem. 2005;280(13):12096–102.
Alikhani M, Roy S, Graves DT. FOXO1 plays an essential role in apoptosis of retinal pericytes. Mol Vis. 2010;16:408–15.
Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–46.
Medema RH, Jaattela M. Cytosolic FOXO1: alive and killing. Nat Cell Biol. 2010;12(7):642–3.
Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FOXO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12(7):665–75.
Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27(50):6398–406.
Lam D, Levraud JP, Luciani MF, Golstein P. Autophagic or necrotic cell death in the absence of caspase and bcl-2 family members. Biochem Biophys Res Commun. 2007;363(3):536–41.
Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. FOXO1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15(11):1307–11.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29.
Dobrin JS, Lebeche D. Diabetic cardiomyopathy: signaling defects and therapeutic approaches. Expert Rev Cardiovasc Ther. 2010;8(3):373–91.
Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53(12):3201–8.
Hattori Y, Matsuda N, Kimura J, Ishitani T, Tamada A, Gando S, Kemmotsu O, Kanno M. Diminished function and expression of the cardiac Na + –Ca2 + exchanger in diabetic rats: implication in Ca2 + overload. J Physiol. 2000;527(Pt 1):85–94.
Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gomez AM. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55(3):608–15.
Takada A, Miki T, Kuno A, Kouzu H, Sunaga D, Itoh T, Tanno M, Yano T, Sato T, Ishikawa S, et al. Role of ER stress in ventricular contractile dysfunction in type 2 diabetes. PLoS One. 2012;7(6):e39893.
Guo W, Jiang T, Lian C, Wang H, Zheng Q, Ma H. QKI deficiency promotes FOXO1 mediated nitrosative stress and endoplasmic reticulum stress contributing to increased vulnerability to ischemic injury in diabetic heart. J Mol Cell Cardiol. 2014;75:131–40.
Liu Z, Zhao N, Zhu H, Zhu S, Pan S, Xu J, Zhang X, Zhang Y, Wang J. Circulating interleukin-1beta promotes endoplasmic reticulum stress-induced myocytes apoptosis in diabetic cardiomyopathy via interleukin-1 receptor-associated kinase-2. Cardiovasc Diabetol. 2015;14(1):125.
Ozcan L, Wong CC, Li G, Xu T, Pajvani U, Park SK, Wronska A, Chen BX, Marks AR, Fukamizu A, et al. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 2012;15(5):739–51.
Kumarswamy R, Lyon AR, Volkmann I, Mills AM, Bretthauer J, Pahuja A, Geers-Knörr C, Kraft T, Hajjar RJ, Macleod KT, et al. SERCA2a gene therapy restores microRNA-1 expression in heart failure via an Akt/FoxO3A-dependent pathway. Eur Heart J. 2012;33(9):1067–75.
Authors’ contributions
All authors participated in drafting and revising the article. All authors read and approved the final manuscript.
Acknowledgements
Authors work was supported in part by the Hong Kong RGC/GRF Grants (17124614M, 17123915M) and in part by the Zhejiang Provincial Top priority first level discipline grant, China. The authors wish to thank Shenzhen IVY-Valued Biotechnology Co., Ltd. for editorial assistance.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kandula, V., Kosuru, R., Li, H. et al. Forkhead box transcription factor 1: role in the pathogenesis of diabetic cardiomyopathy. Cardiovasc Diabetol 15, 44 (2016). https://doi.org/10.1186/s12933-016-0361-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-016-0361-1