Abstract
The coronavirus disease 2019 (COVID-19) is caused by a novel severe acute respiratory syndrome (SARS)–like coronavirus (SARS-CoV-2). Critically ill patients with SARS-COV-2 infection frequently exhibit signs of high oxidative stress and systemic inflammation, which accounts for most of the mortality. Antiviral strategies to inhibit the pathogenic consequences of COVID-19 are urgently required. The nuclear factor erythroid 2–related transcription factor (Nrf2) is a transcription factor that is involved in antioxidant and anti-inflammatory defense in several tissues and cells. This review tries to present an overview of the role of Nrf2 in the treatment of COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the outbreak of the COVID-19 pandemic, the death toll has reached millions and this has undoubtedly had a huge impact on the global healthcare system. According to the latest research from Johns Hopkins University, the COVID-19 pandemic has killed over 6.16 million individuals and infected about 490 million people globally as of April 2022 (data from the WHO). COVID-19 is caused by the infection of SARS-CoV2, belonging to the genus β-coronavirus, and is an enveloped positive-stranded RNA virus, which can infect animals and humans, causing respiratory (Kamal et al. 2020), gastrointestinal (Almeida and Chehter 2020), hepatic (Jothimani et al. 2020), and neurologic diseases (Wang et al. 2020). Once within the host, RNA viruses take advantage of the conditions created by oxidative stress for genome capping and replication, which causes serious illness. Additionally, by choosing specific host factors, positive-strand RNA viruses can change gene expression or reprogram the operation of the host cell’s defense mechanisms. As a result, antioxidant therapy could be used as a treatment for RNA viruses (Pillai et al. 2020).
The role of Nrf2 is twofold: activation of Nrf2 can manifest as both promotion and inhibition of viral disease development. In general, however, activation of Nrf2 has a protective effect on host cells during viral infection. It has been demonstrated in cellular experiments that activation of Nrf2 not only inhibits Zika virus, Ebola virus, and influenza A virus, but has also been shown in in vivo animal studies to reduce mortality and prolong life expectancy after viral infection (Herengt et al. 2021). This suggests that the antiviral effects of Nrf2 activation are preserved across a broad range of viruses. These conserved antiviral effects suggest that Nrf2 controls a broad-acting antiviral program and therefore can be a therapeutic target during viral infections. The mechanisms by which Nrf2 regulates the development of viral pneumonia have been partially elucidated. The following are two different pathways by which viruses reduce intracellular Nrf2, thereby leading to viral pneumonia. In influenza virus (IV), since transcription of the Nrf2 target gene heme oxygenase 1 (HO-1) was found to be enhanced, it is hypothesized that influenza virus strains activate the Nrf2/ARE defense pathway in vitro and in vivo in mice by increasing the nuclear translocation and transcriptional activity of Nrf2. Unlike IV, human respiratory syncytial virus (HRSV) reduces mRNA levels and Nrf2 levels in the nucleus of airway epithelial cells. Indeed, HRSV can lead to Nrf2 deacetylation and subsequent proteasomal degradation, resulting in a downregulated production of antioxidant enzymes (Khomich et al. 2018). Due to the similarity in the pathogenesis of viral pneumonia, we can speculate that Nrf2 may also be involved in the infection process of the RNA virus SARS-Cov-2.
Since there are no mature experimental animal models for SARS-COV-2 infection, it is hard for researchers to completely understand the relationship between COVID-19 and Nrf2 in vivo. However, emerging evidence from clinical practice indicates that Nrf2 signaling suppression is probably associated with COVID-19 pathogenesis. Lung biopsies from COVID-19 patients revealed that genes involved in Nrf2 signaling were highly suppressed (Zhang et al. 2021). Hüseyin Gümüş et al. further found that levels of Nrf2 were significantly decreased in pediatric patients with SARS-COV-2 infection, which in turn resulted in increased levels of total oxidative state (TOS) and total antioxidant state (TAS) (Gumus et al. 2022). Of note, Nrf2 levels in symptomatic patients were even lower than those in asymptomatic ones, suggesting that Nrf2 level might be a potential indicator of disease severity (Zhang et al. 2021). Age, obesity, hyperglycemia, and male gender are identified as risk factors for severe COVID-19 (Chen et al. 2021). Interestingly, the risk factors mentioned above are all related to reduction in Nrf2 levels (Zhang et al. 2015; Vasileva et al. 2020; Baumel-Alterzon et al. 2021; Liu et al. 2021). Nonetheless, it is not clear whether SARS-COV-2 infection induces Nrf2 decrease or Nrf2 decrease renders individuals susceptible to SARS-COV-2 infection. Chances are that both contributed to the pathogenesis of COVID-19. Therefore, further studies are needed to fully elucidate the association between Nrf2 and COVID-19.
We hypothesize that increasing endogenous cellular defenses by targeting the cytoprotective transcription factor Nrf2 would reduce COVID-19-associated inflammation while restoring redox balance and promoting tissue repair.
Pathogenesis of SARS-COV-2 infection
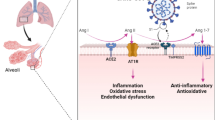
SARS-CoV-2 enters the host cell using a surface receptor, angiotensin-converting enzyme 2 (ACE2) (Yan et al. 2021). The virus adheres to the cell surface by spike proteins (S-proteins). The mutual relationship between S-protein and ACE2 is crucial in the virus’s pathogenic process (Targosz-Korecka et al. 2021). S-protein specifically binds to purified ACE2 receptors via the S1 subunit, including the receptor-binding domain (RBD) (Yang et al. 2020). Since ACE2 no longer converts Ang II to Ang1-7, binding of the viral S protein to ACE2 increases the concentration of Ang II, leading to increased Ang II binding to the angiotensin type 1 receptor (AT1R) and therefore enhanced NADPH oxidase activity. In this condition, the production of ROS (superoxide) and the consumption of NADPH (electron donor) in the cells increase (Singh et al. 2021a). SARS-CoV-2 has 76.5% concordance with SARS-CoV (Zhang et al. 2020a); moreover, both viruses use ACE2 as a cell entry receptor and to initiate subsequent replication (Wu et al. 2012; Wan et al. 2020). In the lung, gene TMPRSS2 expression cleaves the hemagglutinin surface protein of the virus and allows viral fusion and entry into the host cell (Mendonca and Soliman 2020; Davidson et al. 2020). TMPRSS2 can cleave the S-proteins of SARS-CoV-2, which facilitates the fusion of SARS-CoV-2 and cellular membranes, activating SARS-CoV-2 for entry into host cells (Dong et al. 2020; Hoffmann et al. 2020; Sarker et al. 2021). SARS-CoV-2 may have the ability to infect extra-pulmonary organs, and mRNA expression of ACE2 and TMPRSS2 has been observed in the heart, brain, digestive tract, kidney, liver, and other organs (Dong et al. 2020).
Nrf2’s antioxidant roles in COVID-19
Overview of oxidative stress
Oxidative stress (OS) is a biological process that occurs naturally during metabolic processes and plays multiple roles, such as maintaining the balance between oxidant and antioxidant molecules in cells, tissues, and organs (Fernandes et al. 2020; Checconi et al. 2020). Oxidative stress has recently been shown as a key molecular mechanism in the development of COVID-19 (Delgado-Roche and Mesta 2020; Suhail et al. 2020). Oxidative stress is significantly increased during aging process due to the excessive release of ROS (Fernandes et al. 2020; Cecchini and Cecchini 2020). Nrf2 is thought to be a major responder to cellular oxidative stress. Genes induced by Nrf2 are primarily involved in oxidant signaling, antioxidant defense, and drug metabolism and, to a lesser extent, in cell proliferation, metabolism, and proteasomal activity (Saddawi-Konefka et al. 2016). The ability of Nrf2 to produce a rapid protective response to oxidative, inflammatory, and metabolic stress depends on its ubiquitous and constitutive expression in cells (Robledinos-Anton et al. 2019).
ROS is a general term for a series of molecular oxygen derivatives, which are substances that contain oxygen and are reactive. The term “oxidative distress” refers to the molecular damage caused by the elevation of different ROS (Suhail et al. 2020; Sies and Jones 2020). Recent studies have shown that OS also plays a key role in COVID-19 infection (Fernandes et al. 2020; Chernyak et al. 2020). ROS regulates immune regulatory gene expressions in various endothelial cells (Shao et al. 2021). Inflammatory cytokines and ROS act together to activate endothelial pulmonary and epithelial cells. This results in cell contact disintegration, increased permeability, and edematous fluid flow into the alveoli, leading to decreased gas exchange in the lungs. The endothelium is a highly specialized, dynamic, disseminated organ. Because of its broad coverage and the fact that it is the first cell type in the circulation to encounter any pathogen, the endothelium can serve as the primary intravascular sentinel system (Shao et al. 2021). Furthermore, oxidative stress and cytokine storm lead to endothelial cell ROS-dependent apoptosis, which facilitates the release of clotting components and clot formation (Zinovkin and Grebenchikov 2020).
ROS inhibition attenuates viral effects
Viruses need to maintain oxidative stress at an optimal level that is sufficient to support viral metabolism but not to kill any of the host cells. Viruses have evolved to manage ROS levels to their advantage by positively or negatively regulating the Nrf2 pathway (Lee 2018; McCord et al. 2020). Excess reactive oxygen species activate apoptotic signaling pathways to promote normal cell death, so inhibition of ROS attenuates the effects of the virus.
Reactive oxygen is more reactive and toxic through iron overload and iron dysregulation. Free unbound Fe2 + induces ROS formation via the Fenton and Haber–Weiss reactions, which in turn leads to lipid, nucleic acid, and protein destruction. Iron overload is increasingly implicated as a contributor to the pathogenesis of COVID-19 (Habib et al. 2021; Muhoberac 2020). In the experiments of Z Fan et al., it has been shown that Nrf2 reduces cell death due to iron death. One of the key genes regulated by Nrf2 is xCT and it is associated with cytoprotection. Thus, targeting xCT can overcome Nrf2-Keap1-mediated resistance to ferroptosis (Fan et al. 2017). In addition, Nrf2 can also contribute to biodefense against increased reactive oxygen species by mediating the regulation of cytochrome P450 (CYP), a class of heme-containing monooxygenases. Nrf2 depletion has been shown to reduce liver microsomal cytochrome P450 content in the experiments of Takashi Ashino et al. This is mainly due to increased expression of the P450 genes Cyp2a5 and Cyp2b10 by Nrf2 (Ashino et al. 2020). Recent studies have shown that CYP may be involved in the development of lung injury, kidney injury, and liver injury in patients with COVID-19. The expression of CYPs is significantly regulated in the inflammatory process and almost all cytokines downregulate CYPs (Wang et al. 2022).
Nrf2/Keap1/ARE pathway
Nrf2 activates the transcription of genes participating in the defense against ROS (Zhu et al. 2021). Nrf2 participates in a system that is associated with Kelch-like ECH-associated protein 1 (Keap1). In this system, environmental stresses, the generation of both ROS and electrophilic substances, all lead to the decoupling of Keap1 (Fernandes et al. 2020). Under equilibrium conditions, Keap1 forms part of the E3 ubiquitin ligase. It finely regulates its activity by targeting the transcription factor Nrf2 to ubiquitination and proteosome-dependent degradation (Baird and Yamamoto 2020). In the oxidative stress state, Keap1 is inactivated and Nrf2 is released to induce Nrf2-responsive genes (Fernandes et al. 2020; Bousquet et al. 2020; Olagnier et al. 2020). The redox-sensitive twenty-five cysteine residues of Keap1 are shown to have a critical role in controlling the activity of E3 ubiquitin ligase in terms of their oxidant-sensing mechanism. These cysteine residues are essentially very susceptible to conjugation by various ROS inducers. Once conjugated, Keap1-mediated ubiquitination of Nrf2 is severely attenuated. This leads to the liberation of Nrf2 from Keap1-mediated constraints (Thanas et al. 2020). Oxidative stress leads to conformational changes in the Nrf2-Keap1 complex that prevents ubiquitination and allows Nrf2 to translocate to the nucleus, where it forms a heterodimer with small musculoaponeurotic fibrosarcoma (sMaf) and binds the antioxidant response elements (AREs) in the promoter region of genes involved in redox regulation (Checconi et al. 2020). In this way, Nrf2 activation causes the transcription of a vast number of genes that encode antioxidant enzymes and other cytoprotective molecules (Yamamoto et al. 2018) (Fig. 1).
Binding to Nrf2, which is sequestered in the complex, is one way of Keap1 inhibition, resulting in an inability to be ubiquitinated. Modification of several cysteines in Keap1 produces a nonfunctional closed state in which the two Neh2 patterns of Nrf2 (DLG and ETGE) interact with the Keap1 dimer, resulting in an inability to ubiquitinate. As a result, free Keap1 cannot regenerate at a sufficient rate and the newly synthesized Nrf2 avoids Keap1-mediated ubiquitination and subsequent degradation (Robledinos-Anton et al. 2019; Baird and Yamamoto 2020)
Moreover, the thioredoxin system is one of the key regulators of cellular redox homeostasis and includes TRX, thioredoxin reductase (TRXR), and nicotinamide adenine dinucleotide phosphate (NADPH). Oxidized TRX is transformed back to active reduced TRX by TRXR at the cost of NADPH (Jaganjac et al. 2020). Activation of Nrf2 promotes TRXR expression and thus activates TRX, which reduces the disulfide bond between SARS-CoV-2 and ACE2R. This may impair ligand-receptor binding and decrease oxidative stress, decreasing COVID-19 progression (Zhu et al. 2021) (Fig. 2).
The Nrf2/HO-1 signaling axis
Nrf2 produces a large number of cytoprotective and antioxidant enzymes in reaction to oxidative stress. One of these, HO-1, is involved in the metabolism of heme, acting as a catalyst for the cleavage of the porphyrin ring in heme to carbon monoxide, Fe2 + , and bilirubin, and their conversion to bilirubin (Maruyama et al. 2013). Nrf2/HO-1 is a protein that is found in all human cells. Through ARE, it is activated, translocated to the nucleus, and binds to DNA. Nrf2 functions as an antioxidant system modulator, increasing HO-1 expression to reduce oxidative stress (Zhang et al. 2020b).
HO-1 and its products carbon monoxide and bilirubin/biliverdin have been reported to exert beneficial effects on the host during disease and to have a considerable role in the inflammatory process (Espinoza et al. 2017). Free hemoglobin is toxic at the vascular level and raises the oxidant state by increasing the production of free radical species (Balla et al. 1993). HO-1 oxidizes and decomposes heme into ferrous iron, carbon monoxide (CO), and bilirubin (BV). Bilirubin reductase then catalyzes the conversion of BV to bilirubin (BR), which makes BR more electrophilic than BV, thereby relatively increasing the affinity of BR for Keap1-Nrf2. This in turn promotes the induction of Nrf2-dependent antioxidant genes (Singh et al. 2020). Under oxidative stress conditions, BR is converted back into BV, which is then converted back into BR, and the cycle continues, providing crucial protection for endothelial cells (Singh et al. 2020). The antioxidant effect of HO-1 is through the conversion of heme to powerful bilirubinogen oxides and eventually to bilirubin, a powerful antioxidant (Jeong et al. 2019). In addition, Nrf2/HO-1 can reduce oxidative stress by downregulating ROS (Jayawardena et al. 2020). According to data from COVID-19 admissions, smokers have fewer admissions than nonsmokers in the general population. This unexpected finding may be due to coke-induced HO-1 (Hooper 2020).
Nrf2’s anti-inflammatory roles in COVID-19
Several investigations have shown that, contrary to the generally held belief that Nrf2 controls inflammation through redox control, Nrf2 represses the transcriptional upregulation of pro-inflammatory cytokine genes (Kobayashi et al. 2016; Davuljigari et al. 2021).
Several studies have shown that the high inflammatory response induced by SARS-CoV-2 is the main cause of disease progression and death in infected individuals (Merad and Martin 2020; Guven et al. 2021; Santoso et al. 2021). When an antigen like SARS-CoV2 enters the lungs of a healthy, immuno-competent individual, blood-borne monocytes accumulate in the alveoli where they differentiate into M1 macrophages and produce large amounts of pro-inflammatory cytokines (e.g., IL-1, IL-6, and IL-18). These cytokines attract neutrophils, phagocytose antigen(s), and clear infection; resolve tissue damage; and promote recovery in the normal allogeneic state (Calabrese et al. 2021). High expression levels of cytokines such as IL-1B, IFN-γ, IP-10, and MCP-1 have been found in COVID-19 patients (Horowitz and Freeman 2020). Other studies have found that COVID-19 patients in the intensive care unit (ICU) had higher blood levels of cytokines than COVID-19 patients in the general ward (Jayawardena et al. 2020; Hooper 2020). According to the research described above, cytokine storm is positively related to disease severity.
Acute respiratory distress syndrome (ARDS) induced by SARS-CoV-2 can result in alveolar cell injury and pulmonary fibrosis, which are primarily due to a dysregulated host response. The most common symptoms are an increase in pro-inflammatory cytokines (cytokine storm) and a decrease in leukocytes (Cuadrado et al. 2020; Ombrello and Schulert 2021).
SARS-CoV-2 is a virus that can induce apoptosis and local release of pathogen-associated molecular pattern proteins (PAMPs) and various damage-associated molecular pattern proteins (DAMPs). Nrf2 prevents cell and tissue damage and reduces the generation of DAMPs, which are released by necrotic cells and have immunological significance in amplifying the inflammatory response (Tang et al. 2014; Saha et al. 2020; Bime et al. 2021).
Crosstalk in the Nrf2 and NF-kB pathways
The SARS-CoV-2 infection resulted in increased plasma concentrations of inflammatory markers such as C-reactive protein and ferritin; various cytokines such as IL-6, IL-8, TNF-α, and IL-1β; and chemokines such as MCP1, along with an increased neutrophil/lymphocyte ratio (Karki et al. 2021). Recent research has shown a link between Nrf2 and macrophage metabolism, expression of inflammatory mediators, and the NF-κB pathway. (Saha et al. 2020). Nrf2 is abundant in monocytes and granulocytes, suggesting its involvement in immune responses driven by these cell types (Cuadrado et al. 2019).
Similar to Nrf2/ARE signaling, NF-κB is a redox-regulated transcription factor. Activation of the NF-κB pathway, which may eventually lead to stroke or neuropathy associated with cerebral thromboembolism, explains the neurological complications caused by COVID-19 (Bhandari et al. 2021) (Fig. 3).
Oxidative stress induced by viral infection has been shown to play a critical role in activating innate immunity through the NF-κB production of cytokines to defend against pathogenic microorganisms (Narayanan et al. 2014). NF-κB regulates inflammatory responses and cellular injury by inducing the expression of pro-inflammatory cytokines (IL-1, IL-6, TNF-α), COX-2, iNOS, and vascular adhesion molecules. Nrf2 can negatively control the NF-κB signaling pathway through multiple mechanisms, as well as inhibit oxidative stress-mediated NF-κB activation by reducing intracellular ROS levels (Cecchini and Cecchini 2020). On the other hand, lowers free CBP, a transcriptional coactivator of Nrf2, by competing with the CH1-KIX structural domain of CBP, and also increases p65 phosphorylation at Ser276, which hinders CBP binding to Nrf2 (Saha et al. 2020).
Modulation of inflammation by HO-1
Many studies have confirmed the anti-inflammatory properties of HO-1, and thus, induced HO-1 can protect the host in various inflammatory diseases (Park et al. 2021; Gozzelino et al. 2010). HO-1 induction significantly reduced viral multiplication and pulmonary inflammation, as shown by decreased neutrophil infiltration into the airways, decreased cytokine and chemokine production, and decreased T-cell activity (Muhoberac 2020). In contrast, HO-1 deficiency leads to the development of chronic inflammation in mice (Poss and Tonegawa 1997).
Moreover, heme-catalyzed products have been shown to play a direct role in the regulation of inflammatory and immune responses (Wu et al. 2019a). CO has been shown to inhibit the production of various cytokines and to play an important role in HO-1 immunomodulator action in a variety of inflammatory paradigms, including LPS-induced endotoxemia, hyperoxia, ischemia/reperfusion-induced lung damage, and diabetes/hyperglycemia (Costa et al. 2020). Biliverdin and bilirubin show important anti-inflammatory effects and exhibit powerful antioxidant properties, serving as direct scavengers of peroxynitrite (ONOO-) and superoxide (O2-) species (Wegiel and Otterbein 2012).
Therefore, activation of Nrf2 and related pathways (e.g., HO-1) may attenuate the impacts of SARS-CoV-2 infection and lessen the inflammatory response.
Nrf2’s anti-viral roles in COVID-19
The transcription factor Nrf2 is activated after infection. Emanuel Wyler’s research showed that cells with higher numbers of viral transcripts showed relatively high Nrf2 activity, suggesting that Nrf2 may have an antiviral role (Wyler et al. 2019).
Nrf2 stimulates the production of type I interferon
Type I interferon signaling pathway is a key component for the antiviral response of the host innate immune (Li et al. 2020). IFN-I family members include IFN-α and IFN-β (Rojas et al. 2021). After a viral infection, type 1 IFNs are among the first cytokines produced by the host cells to inhibit viral replication (Singh et al. 2020). The production of IFN-I is rapidly triggered by the recognition of pathogen-associated molecular patterns (PAMPs), such as viral nucleic acids, by host sensors (Ribero et al. 2020).
IFNs exhibit broad-spectrum antiviral activity, including antiviral activity against SARS-CoV, according to the literature (Haagmans et al. 2004). It showed that although SARS-CoV-2 kept similar viral replication to SARS-CoV, the former one was more sensitive to IFN-I (Lokugamage et al. 2020). Emily Mantlo’s data demonstrated that both IFN-α and IFN-β treatments in cultured cells were effective against SARS-CoV-2 (Ribero et al. 2020; Mantlo et al. 2020; Thoms et al. 2020). The study by Camilla Gunderstofte studied the function of Nrf2 in the regulation of HSV-induced innate antiviral immunity. Comparison of antiviral gene expression profiles of wild-type and Nrf2 mutant (Nrf2AY/AY) mouse macrophages by RNA sequencing analysis revealed an upregulation of the basal level of the type I interferon-related gene network (Gunderstofte et al. 2019). Autoimmunity of type I IFN immune-deficient B cells is the cause of life-threatening COVID-19 pneumonia (Bastard et al. 2020).
Nrf2 negatively regulates STING
Interferon gene stimulator (STING) is activated by both pathogen and host cytoplasmic DNA, leading to the release of type I interferon (IFN-I) and other cytokines that clear the pathogen through immune cells (Donnelly et al. 2021). Nrf2 regulates STING levels by controlling TMEM173 stability and then downregulating IFN production (Olagnier et al. 2018; Singh et al. 2021b). This has been confirmed experimentally: when primary human monocyte-derived macrophages were silenced by Nrf2, high levels of STING were observed (Singh et al. 2021b). Nrf2 regulates STING levels, which is done by controlling the stability of TMEM173. However, since the lack of Nrf2 in Nrf2-/mouse bone marrow–derived macrophages or Nrf2/Keap1 CRISPR RAW 264.7 macrophages does not interfere with STING expression, the inhibition of STING by Nrf2 is limited to human cells (Olagnier et al. 2018).
Role of Nrf-2/HO-1 axis in antiviral
As mentioned before, HO-1 is an anti-inflammatory, stress-inducible, and cytoprotective enzyme expressed in most cell types in the organism. HO-1 has been reported significant antiviral activity (Chen et al. 2021). Hemin is a natural inducer of the HO-1 gene, and a lot of evidence supports a strong relationship between COVID-19 and haem (Rapozzi et al. 2021; Lechuga et al. 2021). Furthermore, animal studies have revealed that cobalt protoporphyrin-induced HO-1 or its upstream regulatory gene Nrf2 is effective against hepatitis C and B viruses, dengue virus, Zika virus, Ebola virus, human immunodeficiency virus, and human respiratory syncytial virus (Hooper 2020; Wu et al. 2019a). HO-1 expression is essentially regulated by the Keap1/Nrf2 system at the transcriptional level (Seo et al. 2020).
Nrf2 activators for potential therapy of COVID-19
Although Nirmatrelvir plus ritonavir, the first potent drug urgently approved for the treatment of COVID-19, is in clinical use, its phase II/III clinical trials are still ongoing and its effectiveness and safety are still not fully understood (Paumgartten and Oliveira 2020). Therefore, the search for new COVID-19 therapeutic agents remains essential. Nrf2 activators increase Nrf2 levels and enhance the expression of antiviral mediators, which may achieve an “antiviral state” that allows cells to fight viral infection.
PB125®, a phytochemical Nrf2-activating ingredient consisting of three specific, constant ratios of plant extracts, effectively activates Nrf2. It consists of extracts from Rosmarinus officinalis, extracts from Withania somnifera, and extracts from Sophora japonica mixed in a ratio of 15:5:2 (Hybertson et al. 2019). PB125 inhibits viral entry into host cells by downregulating ACE2 and TMPRSS2 mRNA expression in human liver–derived HepG2 cells (McCord et al. 2020). In experiments by Joe M. McCord et al., 36 cytokines in the PB125-treated group, which were strongly upregulated by many respiratory viruses, including the SARS-CoV-2 virus responsible for the current COVID-19 pandemic, were found to be significantly downregulated by PB125 (Hybertson et al. 2019; McCord et al. 2021). The results of these trials suggest that the Nrf2 agonist PB125® may reduce complications in patients with COVID-19.
Currently, the most widely used Nrf2 activator is the fumaric acid ester dimethyl fumarate (DMF) (Robledinos-Anton et al. 2019; Safari et al. 2021). DMF is a potent antioxidant and anti-inflammatory drug that modulates inflammation and oxidative stress, and hence cytoprotection, by upregulating cellular defense mechanisms, such as the expression of phase II antioxidants. The phase II antioxidants mainly involve superoxide dismutase (SOD1), NAD (P) H quinone oxidoreductase-1 (NQO1), and HO-1 (Timpani and Rybalka 2020). In addition, DMF acts through several mechanisms unrelated to Nrf2: inhibition of the NF-κB pathway through binding and activation of IKKβ at Cys-179 reduces the release of NF-κB from its complex in the cytoplasm (NF-κB-IκB), thereby inhibiting downstream pro-inflammatory signaling pathways (Hassan et al. 2020). DMF also inhibits the replication of viruses through IFN-independent mechanisms (Olagnier et al. 2020).
Flavonoids are a family of polyphenolic compounds found in plants with pharmacological properties such as anti-bacterial, anti-fungal, anti-viral antioxidant, anti-inflammatory, and anti-cancer (Ullah et al. 2020). Many flavonoids can activate the transcription factor Nrf2. Not only are epigallocatechin-3-gallate and thymidine Nrf2 inducers, but they are also strong antiviral drugs. They are already marketed as daily supplements (Mendonca and Soliman 2020). Preliminary studies have shown that flavonoids can bind highly affinity to the spike protein, helicase, and protease sites on the ACE2 receptor, causing conformational changes that can inhibit coronavirus entry (Ngwa et al. 2020; Liskova et al. 2021). In the experience of treating COVID-19 with Chinese herbal medicine, a very effective herbal prescription, QFPD, contains 45% flavonoids out of 129 active compounds (Russo et al. 2020). This suggests that flavonoids, which are highly represented in QFPD, may be the active ingredient in the treatment of COVID-19.
Data from Matthew J. Kesic’s study showed that the addition of the potent Nrf2 activator sulforaphane (SFN) and epigallocatechin gallate (EGCG) significantly increased the expression of antiviral mediators even in the absence of or before viral infection (Kesic et al. 2011; Mhatre et al. 2021). Treatment of cells with SFN reverses IL-6 and IL-8 upregulation induced by SARS-CoV-2 spike protein. This represents the possibility that SFN may be able to regulate the release of some key proteins of the COVID-19 “cytokine storm” (Gasparello et al. 2021). A recent study found that EGCG inhibited the replication of HCoV-OC43 (a beta coronavirus, similar to SARS-CoV-2) in a dose-dependent manner and that even 1 µg/mL EGCG significantly reduced HCoV-OC43 protein levels in infected cells (Jang et al. 2021). Recent cellular and animal studies have shown that SFN effectively inhibits SARS-Cov-2 infection and has an effect on the viral pathogenesis of SARS-Cov-2 both from extracellular entry and after intracellular entry. SFN-treated mice also have significantly lower measured viral loads in alveolar fluid, less lung lesions, and less alveolar and peribronchial inflammation (Ordonez et al. 2022). These experiments further validate the role of SFN and EGCG in the fight against SARS-Cov2.
Nrf2 has been shown to have both pro- and anti-cancer effects in cancer, with Nrf2 acting as an oncogenic agent in the early stages of cancer, but promoting the proliferation of cancer cells when Nrf2 is over-activated (Wu et al. 2019b; Huang et al. 2015). It can be hypothesized that Nrf2 is activated as a cellular response during the first stage of COVID-19 infection, while Nrf2 decreases after virus transmission. Although there are few relevant experimental data, the dosage and duration of action of the drug need to be taken into account when approving Nrf2 activators for clinical use.
Concluding remarks and future perspectives
Over the past 3 years, COVID-19 disease caused by the novel coronavirus SARS-CoV-2 has had tremendous health and social impacts and economic losses worldwide.
The various effects of Nrf2, which include antioxidant, anti-inflammatory, and antiviral properties, make this factor a cell fate determinant and a critical role in the systems that regulate cellular transformation and response to viral infection. Overall, all evidence suggests that Nrf2 activation holds promise as a strategy against COVID-19. However, it is advisable to address several important questions before implementing this strategy. More research is needed to detect potential safety issues and determine if continued Nrf2 induction might lead to the development of other diseases, such as cancer. Another pressing issue is the inability of Nrf2 activators to cross the blood–brain barrier for the treatment of neurological complications of COVID-19. In addition, many Nrf2 inhibitors have shown promising results in molecular experiments, and their therapeutic use still needs to be determined in rigorous clinical trials.
Nonetheless, advanced clinical studies in other indications have generated a lot of knowledge on the safety and effectiveness of Nrf2 activators, offering lessons and requirements for their use in randomized clinical trials in patients with COVID-19. If efficient, this treatment technique might be quickly recommended to improve the health of critically ill COVID-19 patients, reducing the requirement for mechanical ventilation and relieving the tremendous pressure now placed on ICUs throughout the world.
References
Ashino T, Yamamoto M, Numazawa S (2020) Nrf2 antioxidative system is involved in cytochrome P450 gene expression and activity: a delay in pentobarbital metabolism in Nrf2-deficient mice. Drug Metab Dispos 48(8):673–680
Baumel-Alterzon S, Katz LS, Brill G, Garcia-Ocana A, Scott DK (2021) Nrf2: the master and captain of beta cell fate. Trends Endocrinol Metab 32(1):7–19
Bhandari R, Khanna G, Kaushik D, Kuhad A (2021) Divulging the intricacies of crosstalk between NF-Kb and Nrf2-Keap1 pathway in neurological complications of COVID-19. Mol Neurobiol 58(7):3347–3361
Bime C, Casanova NG, Nikolich-Zugich J, Knox KS, Camp SM, Garcia JGN (2021) Strategies to DAMPen COVID-19-mediated lung and systemic inflammation and vascular injury. Transl Res 232:37–48
Bousquet J, Cristol JP, Czarlewski W, Anto JM, Martineau A, Haahtela T et al (2020) Nrf2-interacting nutrients and COVID-19: time for research to develop adaptation strategies. Clin Transl Allergy 10(1):58
Calabrese EJ, Kozumbo WJ, Kapoor R, Dhawan G, Lara PC, Giordano J (2021) Nrf2 activation putatively mediates clinical benefits of low-dose radiotherapy in COVID-19 pneumonia and acute respiratory distress syndrome (ARDS): novel mechanistic considerations. Radiother Oncol 160:125–131
Cecchini R, Cecchini AL (2020) SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses 143:110102
Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM et al (2021) Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev 65:101205
Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA, Zinovkin RA (2020) COVID-19 and oxidative stress. Biochem Biokhim 85(12):1543–1553
Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL et al (2019) Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discovery 18(4):295–317
Cuadrado A, Pajares M, Benito C, Jimenez-Villegas J, Escoll M, Fernandez-Gines R et al (2020) Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol Sci 41(9):598–610
Davidson AM, Wysocki J, Batlle D (2020) Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension 76(5):1339–1349
Delgado-Roche L, Mesta F (2020) Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res 51(5):384–387
Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S et al (2020) ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother 131:110678
Donnelly CR, Jiang C, Andriessen AS, Wang K, Wang Z, Ding H et al (2021) STING controls nociception via type I interferon signalling in sensory neurons. Nature 591(7849):275–280
Espinoza JA, González PA, Kalergis AM (2017) Modulation of antiviral immunity by heme oxygenase-1. Am J Pathol 187(3):487–493
Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M, Buchfelder M et al (2017) Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 6(8):e371
Fernandes IG, de Brito CA, Dos Reis VMS, Sato MN, Pereira NZ (2020) SARS-CoV-2 and other respiratory viruses: what does oxidative stress have to do with it? Oxid Med Cell Longev 2020:8844280
Gasparello J, D’Aversa E, Papi C, Gambari L, Grigolo B, Borgatti M et al (2021) Sulforaphane inhibits the expression of interleukin-6 and interleukin-8 induced in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2 spike protein. Phytomedicine 87:153583
Gozzelino R, Jeney V, Soares MP (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354
Gumus H, Erat T, Ozturk I, Demir A, Koyuncu I (2022) Oxidative stress and decreased Nrf2 level in pediatric patients with COVID-19. J Med Virol 94(5):2259–2264
Gunderstofte C, Iversen MB, Peri S, Thielke A, Balachandran S, Holm CK et al (2019) Nrf2 negatively regulates type I interferon responses and increases susceptibility to herpes genital infection in mice. Front Immunol 10:2101
Guven BB, Erturk T, Kompe O, Ersoy A (2021) Serious complications in COVID-19 ARDS cases: pneumothorax, pneumomediastinum, subcutaneous emphysema and haemothorax. Epidemiol Infect 149:e137
Habib HM, Ibrahim S, Zaim A, Ibrahim WH (2021) The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother 136:111228
Hassan SM, Jawad MJ, Ahjel SW, Singh RB, Singh J, Awad SM et al (2020) The Nrf2 activator (DMF) and COVID-19: is there a possible role? Medical Archives 74(2):134–138
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–80.e8
Hooper PL (2020) COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones 25(5):707–710
Horowitz RI, Freeman PR (2020) Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials. Med Hypotheses 143:109851
Jang M, Park R, Park YI, Cha YE, Yamamoto A, Lee JI et al (2021) EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem Biophys Res Commun 547:23–28
Jeong JY, Cha HJ, Choi EO, Kim CH, Kim GY, Yoo YH et al (2019) Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int J Med Sci 16(1):145–155
Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M (2020) COVID-19 and the liver. J Hepatol 73:1231–40
Kesic MJ, Simmons SO, Bauer R, Jaspers I (2011) Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radical Biol Med 51(2):444–453
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H et al (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7:11624
Lee C (2018) Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid Med Cell Longev 2018:1–26
Li JY, Liao CH, Wang Q, Tan YJ, Luo R, Qiu Y et al (2020) The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res 286:198074
Liskova A, Samec M, Koklesova L, Samuel SM, Zhai K, Al-Ishaq RK et al (2021) Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed Pharmacother 138:111430
Liu J, Cui JY, Lu YF, Corton JC, Klaassen CD (2021) Sex-, age-, and race/ethnicity-dependent variations in drug-processing and NRF2-regulated genes in human livers. Drug Metab Dispos 49(1):111–119
Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C (2020) Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 179:104811
Maruyama A, Mimura J, Harada N, Itoh K (2013) Nrf2 activation is associated with Z-DNA formation in the human HO-1 promoter. Nucleic Acids Res 41(10):5223–5234
McCord JM, Hybertson BM, Cota-Gomez A, Gao B (2021) Nrf2 activator PB125(R) as a carnosic acid-based therapeutic agent against respiratory viral diseases, including COVID-19. Free Radical Biol Med 175:56–64
Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20(6):355–362
Mhatre S, Naik S, Patravale V (2021) A molecular docking study of EGCG and theaflavin digallate with the druggable targets of SARS-CoV-2. Comput Biol Med 129:104137
Muhoberac BB (2020) What can cellular redox, iron, and reactive oxygen species suggest about the mechanisms and potential therapy of COVID-19? Front Cell Infect Microbiol 10:569709
Narayanan A, Amaya M, Voss K, Chung M, Benedict A, Sampey G et al (2014) Reactive oxygen species activate NFkappaB (p65) and p53 and induce apoptosis in RVFV infected liver cells. Virology 449:270–286
Olagnier D, Brandtoft AM, Gunderstofte C, Villadsen NL, Krapp C, Thielke AL et al (2018) Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat Commun 9(1):3506
Olagnier D, Farahani E, Thyrsted J, Blay-Cadanet J, Herengt A, Idorn M et al (2020) SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun 11(1):4938
Ombrello MJ, Schulert GS (2021) COVID-19 and cytokine storm syndrome: are there lessons from macrophage activation syndrome? Transl Res 232:1–12
Park C, Lee H, Kwon C-Y et al (2021) Loganin inhibits lipopolysaccharide-induced inflammation and oxidative response through the activation of the Nrf2/HO-1 signaling pathway in RAW264.7 Macrophages. Biol Pharm Bull. 44:875–83
Paumgartten FJR, Oliveira A (2020) Off label, compassionate and irrational use of medicines in COVID-19 pandemic, health consequences and ethical issues. Ciencia & Saude Coletiva 25(9):3413–3419
Pillai AB, JeanPierre AR, Mariappan V, Ranganadin P, Rao SR (2022) Neutralizing the free radicals could alleviate the disease severity following an infection by positive strand RNA viruses. Cell Stress Chaperones 27(3):189–95
Poss KD (1997) Tonegawa S (1997) Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94:10919–24
Rapozzi V, Juarranz A, Habib A, Ihan A, Strgar R (2021) Is haem the real target of COVID-19? Photodiagn Photodyn Ther 35:102381
Ribero MS, Jouvenet N, Dreux M et al (2020) Interplay between SARS-CoV-2 and the type I interferon response. PLos Pathog 16(7):e1008737
Robledinos-Anton N, Fernandez-Gines R, Manda G, Cuadrado A (2019) Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev 2019:9372182
Rojas JM, Alejo A, Martin V, Sevilla N (2021) Viral pathogen-induced mechanisms to antagonize mammalian interferon (IFN) signaling pathway. Cell Mol Life Sci 78(4):1423–1444
Russo M, Moccia S, Spagnuolo C, Tedesco I, Russo GL (2020) Roles of flavonoids against coronavirus infection. Chem Biol Interact 328:109211
Saddawi-Konefka R, Seelige R, Gross ET, Levy E, Searles SC, Washington A Jr et al (2016) Nrf2 induces IL-17D to mediate tumor and virus surveillance. Cell Rep 16(9):2348–2358
Safari A, Khodabandeh Z, Borhani-Haghighi A (2021) Dimethyl fumarate can enhance the potential therapeutic effects of epidermal neural crest stem cells in COVID-19 patients. Stem Cell Rev Rep 17(1):300–301
Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B (2021) Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med 44:352–357
Sarker J, Das P, Sarker S, Roy AK, Momen A (2021) A review on expression, pathological roles, and inhibition of TMPRSS2, the serine protease responsible for SARS-CoV-2 spike protein activation. Scientifica 2021:2706789
Seo HY, Lee SH, Lee JH, Hwang JS, Kim MK, Jang BK (2020) Kahweol activates the Nrf2/HO-1 pathway by decreasing Keap1 expression independently of p62 and autophagy pathways. PLoS ONE 15(10):e0240478
Shao Y, Saredy J, Xu K, Sun Y, Saaoud F, Drummer CT et al (2021) Endothelial immunity trained by coronavirus infections, DAMP stimulations and regulated by anti-oxidant NRF2 may contribute to inflammations, myelopoiesis, COVID-19 cytokine storms and thromboembolism. Front Immunol 12:653110
Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21(7):363–383
Singh D, Wasan H, Reeta KH (2020) Heme oxygenase-1 modulation: a potential therapeutic target for COVID-19 and associated complications. Free Radical Biol Med 161:263–271
Singh H, Choudhari R, Nema V, Khan AA (2021a) ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb Pathog 150:104621
Suhail S, Zajac J, Fossum C, Lowater H, McCracken C, Severson N et al (2020) Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J 39(6):644–656
Tang W, Jiang YF, Ponnusamy M, Diallo M (2014) Role of Nrf2 in chronic liver disease. World J Gastroenterol 20(36):13079–13087
Targosz-Korecka M, Kubisiak A, Kloska D, Kopacz A, Grochot-Przeczek A, Szymonski M (2021) Endothelial glycocalyx shields the interaction of SARS-CoV-2 spike protein with ACE2 receptors. Sci Rep 11(1):12157
Thoms M, Buschauer R, Ameismeier M (2020) Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Coronavirus. 369:1249–1255
Vasileva LV, Savova MS, Amirova KM, Dinkova-Kostova AT, Georgiev MI (2020) Obesity and NRF2-mediated cytoprotection: where is the missing link? Pharmacol Res 156:104760
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama 323(11):1061–1069
Wang G, Xiao B, Deng J, Gong L, Li Y, Li J et al (2022) The role of cytochrome P450 enzymes in COVID-19 pathogenesis and therapy. Front Pharmacol 13:791922
Wegiel B, Otterbein LE (2012) Go green: the anti-inflammatory effects of biliverdin reductase. Front Pharmacol 3:47
Wu K, Peng G, Wilken M, Geraghty RJ, Li F (2012) Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. Biol Chem 287:8904–11
Wu B, Wu Y, Tang W (2019a) Heme catabolic pathway in inflammation and immune disorders. Front Pharmacol 10:825
Wu S, Lu H, Bai Y (2019b) Nrf2 in cancers: a double-edged sword. Cancer Med 8(5):2252–2267
Wyler E, Franke V, Menegatti J, Kocks C, Boltengagen A, Praktiknjo S et al (2019) Single-cell RNA-sequencing of herpes simplex virus 1-infected cells connects NRF2 activation to an antiviral program. Nat Commun 10(1):4878
Yamamoto M, Kensler TW, Motohashi H (2018) The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 98(3):1169–1203
Yan R, Wang R, Ju B, Yu J, Zhang Y, Liu N et al (2021) Structural basis for bivalent binding and inhibition of SARS-CoV-2 infection by human potent neutralizing antibodies. Cell Res 31(5):517–525
Yang J, Petitjean SJL, Koehler M, Zhang Q, Dumitru AC, Chen W et al (2020) Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun 11(1):4541
Zhang H, Davies KJA, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radical Biol Med 88(Pt B):314–336
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020a) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4):586–590
Zhang X, Yu Y, Lei H, Cai Y, Shen J, Zhu P et al (2020b) The Nrf-2/HO-1 signaling axis: a ray of hope in cardiovascular diseases. Cardiol Res Pract 2020:5695723
Zhang Z, Zhang X, Bi K, He Y, Yan W, Yang CS et al (2021) Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci Technol 114:11–24
Zhu Z, Zheng Z, Liu J (2021) Comparison of COVID-19 and lung cancer via reactive oxygen species signaling. Front Oncol 11:708263
Zinovkin RA, Grebenchikov OA (2020) Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochem Biokhim 85(7):833–837
Kamal M, Abo Omirah M, Hussein A, Saeed H (2020) Assessment and characterisation of post‐COVID‐19 manifestations. Int J Clin Pract 75(3)
Almeida JFMd, Chehter EZ (2020) COVID-19 and the gastrointestinal tract: what do we already know?
Herengt A, Thyrsted J, Holm CK (2021) NRF2 in viral infection. Antioxidants 10(9)
Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV (2018) Redox biology of respiratory viral infections. Viruses 10(8)
Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94(7)
Mendonca P, Soliman KFA (2020) Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants 9(8)
Checconi P, De Angelis M, Marcocci ME, Fraternale A, Magnani M, Palamara AT, et al (2020) Redox-modulating agents in the treatment of viral infections. Int J Mol Sci 21(11)
McCord JM, Hybertson BM, Cota-Gomez A, Geraci KP, Gao B (2020) Nrf2 activator PB125((R)) as a potential therapeutic agent against COVID-19. Antioxidants 9(6)
Baird L, Yamamoto M (2020) The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol 40(13)
Thanas C, Ziros PG, Chartoumpekis DV, Renaud CO, Sykiotis GP (2020) The Keap1/Nrf2 signaling pathway in the thyroid-2020 update. Antioxidants 9(11)
Jaganjac M, Milkovic L, Sunjic SB, Zarkovic N (2020) The NRF2, thioredoxin, and glutathione system in tumorigenesis and anticancer therapies. Antioxidants 9(11)
Balla J, Jacob HS, Balla G, Nath K, Eaton JW et al (1993) Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. 90
Jayawardena TU, Sanjeewa KKA, Lee HG, Nagahawatta DP, Yang HW, Kang MC et al (2020) Particulate matter-induced inflammation/oxidative stress in macrophages: fucosterol from Padina boryana as a potent protector, activated via NF-kappaB/MAPK pathways and Nrf2/HO-1 involvement. Marine Drugs 18(12)
Davuljigari CB, Ekuban FA, Zong C, Fergany AAM, Morikawa K, Ichihara G (2021) Nrf2 activation attenuates acrylamide-induced neuropathy in mice. Int J Mol Sci 22(11)
Saha S, Buttari B, Panieri E, Profumo E, Saso L (2020) An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 25(22)
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, et al (2021) Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 184(1):149–68 e17
Costa DL, Amaral EP, Andrade BB, Sher A (2020) Modulation of inflammation and immune responses by heme oxygenase-1: implications for infection with intracellular pathogens. Antioxidants 9(12)
Haagmans BL, Kuiken T, Martina BE et al (2004) Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques
Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M et al (2020) Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol 94(23)
Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y et al (2020) Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370(6515)
Singh E, Matada GSP, Abbas N, Dhiwar PS, Ghara A, Das A (2021b) Management of COVID-19-induced cytokine storm by Keap1-Nrf2 system: a review. Inflammopharmacology
Chen WC, Wei CK, Hossen M, Hsu YC, Lee JC (2021) (E)-Guggulsterone inhibits dengue virus replication by upregulating antiviral interferon responses through the induction of heme oxygenase-1 expression. Viruses 13(4)
Lechuga GC, Souza-Silva F, Sacramento CQ, Trugilho MRO, Valente RH, Napoleao-Pego P et al (2021) SARS-CoV-2 proteins bind to hemoglobin and its metabolites. Int J Mol Sci 22(16)
Hybertson BM, Gao B, Bose S, McCord JM (2019) Phytochemical combination PB125 activates the Nrf2 pathway and induces cellular protection against oxidative injury. Antioxidants 8(5)
Timpani CA, Rybalka E (2020) Calming the (cytokine) storm: dimethyl fumarate as a therapeutic candidate for COVID-19. Pharmaceuticals 14(1)
Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG et al (2020) Important flavonoids and their role as a therapeutic agent. Molecules 25(22)
Ngwa W, Kumar R, Thompson D, Lyerly W, Moore R, Reid TE et al (2020) Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules 25(11)
Ordonez AA, Bullen CK, Villabona-Rueda AF, Thompson EA, Turner ML, Merino VF, et al (2022) Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun Biol 5(1)
Huang Y, Li W, Su Z-y, Kong A-NT (2015) The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem 26(12):1401–13
Funding
This paper was supported by the National Natural Science Foundation of China (No.81974079), National Key R&D Program of China (No.2019YFE0190800), Key R&D Program of Hunan province (No.2020SK30291), and National Natural Science Foundation of Hunan province (No.2022JJ30825).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Ma, J. & Jiang, Y. Transcription factor Nrf2 as a potential therapeutic target for COVID-19. Cell Stress and Chaperones 28, 11–20 (2023). https://doi.org/10.1007/s12192-022-01296-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-022-01296-8