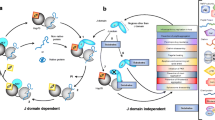
Abstract
Hsp70 chaperone systems are very versatile machines present in nearly all living organisms and in nearly all intracellular compartments. They function in many fundamental processes through their facilitation of protein (re)folding, trafficking, remodeling, disaggregation, and degradation. Hsp70 machines are regulated by co-chaperones. J-domain containing proteins (JDPs) are the largest family of Hsp70 co-chaperones and play a determining role functionally specifying and directing Hsp70 functions. Many features of JDPs are not understood; however, a number of JDP experts gathered at a recent CSSI-sponsored workshop in Gdansk (Poland) to discuss various aspects of J-domain protein function, evolution, and structure. In this report, we present the main findings and the consensus reached to help direct future developments in the field of Hsp70 research.
Similar content being viewed by others
References
Baaklini I, Wong MJ, Hantouche C, Patel Y, Shrier A, Young JC (2012) The DNAJA2 substrate release mechanism is essential for chaperone-mediated folding. J Biol Chem 287(50):41939–41954
Behnke J, Mann MJ, Scruggs FL, Feige MJ, Hendershot LM (2016) Members of the Hsp70 family recognize distinct types of sequences to execute ER quality control. Mol Cell 63(5):739–752
Bracher A, Verghese J (2015) The nucleotide exchange factors of Hsp70 molecular chaperones. Front Mol Biosci 2:10
Cheetham ME, Caplan AJ (1998) Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones 3(1):28–36
Chen KC, Qu S, Chowdhury S, Noxon IC, Schonhoft JD, Plate L, Powers ET, Kelly JW, Lander GC, Wiseman RL (2017) The endoplasmic reticulum HSP40 co-chaperone ERdj3/DNAJB11 assembles and functions as a tetramer. EMBO J 36(15):2296–2309
Craig EA, Marszalek J (2017) How do J-proteins get Hsp70 to do so many different things? Trends Biochem Sci 42(5):355–368
Craig EA (2018) Hsp70 at the membrane: driving protein translocation. BMC Biol 16(1):11
Cyr DM, Langer T, Douglas MG (1994) DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci 19(4):176–181
Delewski W, Paterkiewicz B, Manicki M, Schilke B, Tomiczek B, Ciesielski SJ, Nierzwicki L, Czub J, Dutkiewicz R, Craig EA, Marszalek J (2016) Iron–sulfur cluster biogenesis chaperones: evidence for emergence of mutational robustness of a highly specific protein–protein interaction. Mol Biol Evol 33(3):643–656
Finka A, Mattoo RU, Goloubinoff P (2011) Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells. Cell Stress Chaperones 16(1):15–31
Gowda NKC, Kaimal JM, Kityk R, Daniel C, Liebau J, Öhman M, Mayer MP, Andréasson C (2018) Nucleotide exchange factors Fes1 and HspBP1 mimic substrate to release misfolded proteins from Hsp70. Nat Struct Mol Biol 25(1):83–89
Grove DE, Fan CY, Ren HY, Cyr DM (2011) The endoplasmic reticulum-associated Hsp40 DNAJB12 and Hsc70 cooperate to facilitate RMA1 E3-dependent degradation of nascent CFTRDeltaF508. Mol Biol Cell 22(3):301–314
Hageman J, Kampinga HH (2009) Computational analysis of the human HSPH/HSPA/DNAJ family and cloning of a human HSPH/HSPA/DNAJ expression library. Cell Stress Chaperones 14(1):1–21
Hageman J, Rujano MA, van Waarde MA, Kakkar V, Dirks RP, Govorukhina N, Oosterveld-Hut HM, Lubsen NH, Kampinga HH (2010) A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol Cell 37(3):355–369
Hassdenteufel S, Johnson N, Paton AW, Paton JC, High S, Zimmermann R (2018) Chaperone-mediated Sec61 channel gating during ER import of small precursor proteins overcomes Sec61 inhibitor-reinforced energy barrier. Cell Rep 23(5):1373–1386
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11(8):579–592
Kakkar V, Meister-Broekema M, Minoia M, Carra S, Kampinga HH (2014) Barcoding heat shock proteins to human diseases: looking beyond the heat shock response. Dis Model Mech 7(4):421–434
Kakkar V, Mansson C, de Mattos EP, Bergink S, van der Zwaag M, van Waarde MAWH, Kloosterhuis NJ, Melki R, van Cruchten RTP, Al-Karadaghi S, Arosio P, Dobson CM, Knowles TPJ, Bates GP, van Deursen JM, Linse S, van de Sluis B, Emanuelsson C, Kampinga HH (2016) The S/T-rich motif in the DNAJB6 chaperone delays polyglutamine aggregation and the onset of disease in a mouse model. Mol Cell 62(2):272–283
Kirstein J, Arnsburg K, Scior A, Szlachcic A, Guilbride DL, Morimoto RI, Bukau B, Nillegoda NB (2017) In vivo properties of the disaggregase function of J-proteins and Hsc70 in Caenorhabditis elegans stress and aging. Aging Cell 16:1414–1424
Kityk R, Kopp J, Mayer MP (2018) Molecular mechanism of J-domain-triggered ATP hydrolysis by Hsp70 chaperones. Mol Cell 69(2):227–237
Labbadia J, Novoselov SS, Bett JS, Weiss A, Paganetti P, Bates GP, Cheetham ME (2012) Suppression of protein aggregation by chaperone modification of high molecular weight complexes. Brain 135(Pt 4):1180–1196
Li K, Jiang Q, Bai X, Yang YF, Ruan MY, Cai SQ (2017) Tetrameric assembly of K(+) channels requires ER-located chaperone proteins. Mol Cell 65(1):52–65
Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M (1991) Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A 88:2874–2878
Linxweiler M, Schick B, Zimmermann R (2017) Let’s talk about Secs: Sec61, Sec62 and Sec63 in signal transduction, oncology and personalized medicine. Signal Transduct Target Ther 2:17002
Månsson C, van Cruchten RTP, Weininger U, Yang X, Cukalevski R, Arosio P, Dobson CM, Knowles T, Akke M, Linse S, Emanuelsson C, Conserved S (2018) T residues of the human chaperone DNAJB6 are required for effective inhibition of Aβ42 amyloid fibril formation. Biochemistry 57(32):4891–4902
Malinverni D, Jost Lopez A, De Los Rios P, Hummer G, Barducci A (2017) Modeling Hsp70/Hsp40 interaction by multi-scale molecular simulations and coevolutionary sequence analysis. Elife:6
Mayer MP (2018) Intra-molecular pathways of allosteric control in Hsp70s. Philos Trans R Soc Lond Ser B Biol Sci 373(1749):20170183
Melnyk A, Rieger H, Zimmermann R (2015) Co-chaperones of the mammalian endoplasmic reticulum. Subcell Biochem 78:179–200
Mok SA, Condello C, Freilich R, Gillies A, Arhar T, Oroz J, Kadavath H, Julien O, Assimon VA, Rauch JN, Dunyak BM, Lee J, Tsai FTF, Wilson MR, Zweckstetter M, Dickey CA, Gestwicki JE (2018) Mapping interactions with the chaperone network reveals factors that protect against tau aggregation. Nat Struct Mol Biol 25(5):384–393
Nillegoda NB, Kirstein J, Szlachcic A, Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A, Aebersold R, Guilbride DL, Wade RC, Morimoto RI, Mayer MP, Bukau B (2015) Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524(7564):247–251
Nillegoda NB, Stank A, Malinverni D, Alberts N, Szlachcic A, Barducci A, De Los Rios P, Wade RC, Bukau B. Evolution of an intricate J-protein network driving protein disaggregation in eukaryotes. elife 2017;6. pii: e24560. https://doi.org/10.7554/eLife.24560
Nillegoda NB, Wentink AS, Bukau B (2018) Protein disaggregation in multicellular organisms. Trends Biochem Sci 43(4):285–300
Perales-Calvo J, Muga A, Moro F (2010) Role of DnaJ G/F-rich domain in conformational recognition and binding of protein substrates. J Biol Chem 285(44):34231–34239
Perrody E, Cirinesi AM, Desplats C, Keppel F, Schwager F, Tranier S, Georgopoulos C, Genevaux P (2012) A bacteriophage-encoded J-domain protein interacts with the DnaK/Hsp70 chaperone and stabilizes the heat-shock factor σ32 of Escherichia coli. PLoS Genet 8(11):e1003037. https://doi.org/10.1371/journal.pgen.1003037
Pulido P, Leister D (2018) Novel DNAJ-related proteins in Arabidopsis thaliana. New Phytol 217(2):480–490
Rauch JN, Zuiderweg ER, Gestwicki JE (2016) Non-canonical interactions between heat shock cognate protein 70 (Hsc70) and Bcl2-associated Anthanogene (BAG) co-chaperones are important for client release. J Biol Chem 291(38):19848–19857
Rosam M, Krader D, Nickels C, Hochmair J, Back KC, Agam G, Barth A, Zeymer C, Hendrix J, Schneider M, Antes I, Reinstein J, Lamb DC, Buchner J (2018) Bap (Sil1) regulates the molecular chaperone BiP by coupling release of nucleotide and substrate. Nat Struct Mol Biol 25(1):90–100
Rosenzweig R, Sekhar A, Nagesh J, Kay LE. Promiscuous binding by Hsp70 results in conformational heterogeneity and fuzzy chaperone-substrate ensembles. elife 2017;6. pii: e28030. https://doi.org/10.7554/eLife.28030
Ruggieri A, Saredi S, Zanotti S, Pasanisi MB, Maggi L, M1 M (2016) DNAJB6 myopathies: focused review on an emerging and expanding group of myopathies. Front Mol Biosci 3(63) eCollection 2016
Sahi C, Kominek J, Ziegelhoffer T, Yu HY, Baranowski M, Marszalek J, Craig EA (2013) Sequential duplications of an ancient member of the DnaJ-family expanded the functional chaperone network in the eukaryotic cytosol. Mol Biol Evol 30(5):985–998
Scior A, Buntru A, Arnsburg K, Ast A, Iburg M, Juenemann K, Pigazzini ML, Mlody B, Puchkov D, Priller J, Wanker EE, Prigione A, Kirstein J (2018) Complete suppression of Htt fibrilization and disaggregation of Htt fibrils by a trimeric chaperone complex. EMBO J 37(2):282–299
Sharma SK, De los Rios P, Christen P, Lustig A, Goloubinoff P (2010) The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat Chem Biol 6(12):914–920
Soderberg CAG, Mannsson C, Bernfur K, Rutsdottir G, Harmark J, Rajan S, Al-Karadaghi S, Rasmussen M, Hajrup P, Hebert H, Emanuelsson C (2018) Structural modelling of the DNAJB6 oligomeric chaperone shows a peptide-binding cleft lined with conserved S/T-residues at the dimer interface. Sci Rep 8(1):5199
Swain JF, Gierasch LM (2006) The changing landscape of protein allostery. Curr Opin Struct Biol 16(1):102–108
Taylor IR, Dunyak BM, Komiyama T, Shao H, Ran X, Assimon VA, Kalyanaraman C, Rauch JN, Jacobson MP, Zuiderweg ERP, Gestwicki JE (2018) High-throughput screen for inhibitors of protein-protein interactions in a reconstituted heat shock protein 70 (Hsp70) complex. J Biol Chem 293(11):4014–4025
Ushioda R, Miyamoto A, Inoue M, Watanabe S, Okumura M, Maegawa KI, Uegaki K, Fujii S, Fukuda Y, Umitsu M, Takagi J, Inaba K, Mikoshiba K, Nagata K (2016) Redox-assisted regulation of Ca2+ homeostasis in the endoplasmic reticulum by disulfide reductase ERdj5. Proc Natl Acad Sci U S A 113(41):E6055–E6063
Wall D, Zylicz M, Georgopoulos C (1995) The conserved G/F motif of the DnaJ chaperone is necessary for the activation of the substrate binding properties of the DnaK chaperone. J Biol Chem 270(5):2139–2144
Wawrzynow B, Zylicz A, Zylicz M (2018) Chaperoning the guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action. Biochim Biophys Acta Rev Cancer 1869(2):161–174
Yan W, Craig EA (1999) The glycine-phenylalanine-rich region determines the specificity of the yeast Hsp40 Sis1. Mol Cell Biol 19(11):7751–7758
Zarouchlioti C, Parfitt DA, Li W, Gittings LM, Cheetham ME (2018) DNAJ proteins in neurodegeneration: essential and protective factors. Philos Trans R Soc Lond Ser B Biol Sci 373(1738):20160534
Zhang Y, Sinning I, Rospert S (2017) Two chaperones locked in an embrace: structure and function of the ribosome-associated complex RAC. Nat Struct Mol Biol 24(8):611–619
Zuiderweg ER, Bertelsen EB, Rousaki A, Mayer MP, Gestwicki JE, Ahmad A (2013) Allostery in the Hsp70 chaperone proteins. Top Curr Chem 328:99–153
Funding
The organizers would like to thank the Cell Stress Society International (CSSI) for their financial support of the workshop. We also thank the Rector of the University of Gdansk, the Dean of the Intercollegiate Faculty of Biotechnology, University of Gdansk and Medical University of Gdansk, and The University Medical Center Groningen for financial support. During organization of this workshop JM was supported by Polish National Science Center Grant DEC-2012/06/A/NZ1/00002.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 1089 kb)
Rights and permissions
About this article
Cite this article
Kampinga, H.H., Andreasson, C., Barducci, A. et al. Function, evolution, and structure of J-domain proteins. Cell Stress and Chaperones 24, 7–15 (2019). https://doi.org/10.1007/s12192-018-0948-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-018-0948-4