Abstract
Hepatitis is inflammation of the liver, and one of its types, hepatitis B, is a contagious infection. Using mathematical models, the nature of the spread of the Hepatitis B virus can be predicted. In the present paper, a hepatitis B epidemic model with a Beddington–DeAngelis type incidence rate and a constant vaccination rate is considered. Some dynamical properties of this model, such as non-negativity, boundedness character, the basic reproduction number \(\mathcal {R}_0\), stability nature, and the bifurcation phenomenon, are investigated. By the Bendixson theorem, it is demonstrated that the disease-free equilibrium is globally asymptotically stable. It is shown that a transcritical bifurcation phenomenon occurs when \(\mathcal {R}_0=1\). It is concluded that the endemic equilibrium is globally asymptotically stable when \(\mathcal {R}_0>1\), by utilizing Dulac’s criteria. Also, a discrete system of difference equations is obtained by constructing a non-standard finite difference (NSFD) scheme for the continuous model. It is shown that the solutions of this discrete system are dynamically consistent for all finite step sizes. The theoretical results obtained are also supported and visualized by numerical simulations. These simulations also demonstrate that the NSFD scheme produces much more efficient results than the Euler or RK4 schemes, as shown in the theoretical results obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Outbreaks of infectious diseases pose a significant threat to human health. One of the most dangerous epidemic diseases that causes a large number of deaths worldwide is hepatitis B. Hepatitis B infection is a viral disease caused by the hepatitis B virus (HBV), which primarily affects the liver. It is a significant global health issue. If left undetected and untreated, this disease can lead to cirrhosis, liver cancer, or liver failure, resulting in substantial morbidity and mortality worldwide. Notably, it is associated with approximately 800,000 deaths annually, primarily from liver cancer and cirrhosis, making it a significant global threat. Additionally, it is estimated that there are over 350 million chronic HBV carriers worldwide. In chronic carriers, HBV persists within the host’s body for an extended period, posing a significant risk to overall health. While many individuals with carrier hepatitis never experience an acute infection, hepatic fibrosis can develop, eventually leading to liver failure. Medical research has revealed that HBV infection is responsible for approximately \(80\%\) of all primary liver cancer cases, highlighting the urgent need for effective techniques to predict and eradicate HBV. Advanced preventive treatments are available to prevent the spread of HBV. Among these measures, routine vaccination programs have been highly effective in controlling the spread of HBV. The virus spreads through contact with an infected person’s blood, semen, or other bodily fluids (see [1,2,3]).
In response to this urgent need, an effective technique is highly necessary to predict and eradicate HBV. Mathematical modeling, like many other epidemiological diseases, is one of the most effective tools used for hepatitis B. Extensive research has been conducted to develop deterministic and stochastic mathematical models for the spread dynamics of infectious diseases (see [2,3,4,5,6,7,8,9,10,11,12,13,14,15] and references therein). The classical SIR model, introduced as early as 1927 by Kermack and McKendrick, remains one of the oldest and most extensively used models in the field of mathematical epidemiology (see [16]). In this study, we will formulate a deterministic model for the transmission of HBV using the classical SIR model concept.
1.1 Mathematical tools and literature survey
In mathematical epidemiology, models depend on an incidence rate which represents the number of individuals who become infected per unit of time, and plays a crucial role in ensuring that the models accurately capture the qualitative dynamics of disease transmission. In the classical SIR model proposed by Kermack and McKendrick, the incidence rate is given by \(\beta SI\), where \(\beta \) represents the infection rate and S and I represent susceptible and infected individuals, respectively. This type of incidence rate is commonly known as mass action incidence or bilinear incidence (see [13, 14, 16, 17]). A widely used alternative to the bilinear incidence rate in epidemic models is the standard incidence rate \(\frac{\beta SI}{N}\), where N represents the total population size. While the bilinear and standard incidence rates coincide when the total population size remains constant, they diverge in cases where the total population size varies. Bilinear incidence is employed in diseases where disease-related contact rises as the population size increases. Standard incidence is utilized in diseases where the contact rate cannot continually increase and is constrained, even with an increase in the population size (see [18]). It is important to note that, bilinear incidence rate might yield unrealistic outcomes for large populations since it implies that the number of individuals getting infected per unit of time increases as the number of susceptible individuals increases, which may not be a realistic assumption. To address this concern, various alternative incidence rates have been proposed in the literature. For instance, in 1978, Anderson and May [19] introduced a saturated incidence rate in the form of \(\frac{\beta SI}{1+\alpha _1S}\), where the saturation factor \(\alpha _1\) represents the effect of epidemic control. Another approach suggested by Capasso and Serio in [20] involves a saturated incidence rate of \(\frac{\beta SI}{1+\alpha _2 I}\), where \(\frac{1}{1+\alpha _2 I}\) accounts for the inhibitory effect resulting from behavioral changes among susceptible individuals or the crowding effect caused by infectives. These alternative approaches aim to overcome the limitations of the bilinear incidence rate and offer a more realistic representation of the infection dynamics. These saturated incidence rates have been utilized by several authors in epidemiological models (see [2, 15]). In 1975, Beddington in [21] and DeAngelis in [22] independently introduced the non-linear incidence rate known as the Beddington–DeAngelis type, expressed as \(\frac{\beta SI}{1+\alpha _1S+\alpha _2I}\). Many researchers have employed this non-linear incidence rate in subsequent studies to describe their epidemiological models (see [23,24,25]). Beddington–DeAngelis type incidence rate can be expressed in three different forms:
-
(i)
\(\beta SI\) when both \(\alpha _1\) and \(\alpha _2\) are equal to zero,
-
(ii)
\(\frac{\beta SI}{1+\alpha _1S}\) when \(\alpha _2\) is equal to zero,
-
(iii)
\(\frac{\beta SI}{1+\alpha _2I}\) when \(\alpha _1\) is equal to zero.
Therefore the Beddington–DeAngelis type incidence rate is a generalization of the bilinear and saturated incidence rates. This is because the Beddington–DeAngelis type incidence rate takes into account inhibition effects such as susceptible individuals taking preventive measures and infected individuals undergoing treatment (\(\alpha _1\) and \(\alpha _2\) parameters). Also, vaccination plays a pivotal role in the prevention of infectious diseases from spreading. Including the vaccination parameter in the model may be necessary to develop models suitable for the disease. Numerous researchers in the literature have extensively investigated mathematical models with vaccination (see [2, 3, 17, 18, 26, 27]).
Epidemiological models are often formulated as systems of non-linear differential or difference equations. When a model represented by a nonlinear system of differential equations is given, this model can be discretized using certain methods. It is important to ensure that the discretized model retains as many dynamical properties of the continuous model as possible. For the purpose of discretization, Euler and Runge–Kutta methods, along with numerous other finite-difference techniques, are commonly used. However, these methods can give rise to certain undesirable dynamical behaviors. These include convergence to incorrect equilibrium points or periodic cycles, as well as numerical instabilities (see [15, 28, 29]). Mickens introduced an innovative approach known as the non-standard finite difference (NSFD) method to address these issues (see [30, 31]). If a property P is observed in both a differential equation and/or its solutions, as well as in the corresponding discrete equation and/or its solutions, it is stated that these two equations are dynamically consistent in terms of property P. For a mathematical model and its finite difference discretization to be considered valid, it is crucial that they are dynamically consistent with each other. The NSFD method has found applications in a wide range of problems, where it has been observed that the resulting discrete models successfully retain the dynamical properties of the corresponding continuous models (see [13, 15, 29, 32,33,34]).
In [23, 24], Kaddar proposed a delayed SIR model with the Beddington–DeAngelis incidence rate given by the system
where \(\Lambda \) denotes the recruitment rate, \(\beta \) is the disease transmission rate, \(\mu \) is the natural death rate. \(\nu \) is the proportion of the infectives that are treated per unit of time, \(\sigma \) is the death rate induced by the disease. Kaddar analyzed some dynamics of the model (1.1). In [15], the model (1.1) was discretized by using a non-standard finite difference scheme using Mickens’s idea, and the authors showed that the discretized model is dynamically consistent with continuous model in terms of some dynamical properties.
In [2], the authors proposed and analyzed an SIR model for the control of HBV spreading by the system
where p is the proportion of the susceptibles that are vaccinated per unit of time. In [3], the authors constructed a non-standard difference scheme for the model (1.2):
They reached dynamically consistent results with the continuous model under some assumptions on the time step size value h. In [33], the authors discretized the model (1.2) by a different approach:
Here \(\varphi \) is a function of h and called the denominator function. The authors have dynamically consistent results with the continuous model without any constraint on the value of h.
In this study, motivated by the papers discussed above, an HBV model incorporating the Beddington–DeAngelis type incidence rate, constant vaccination, and treatment rates has been presented. Firstly, a dynamical analysis has been conducted to determine the existence of positive solutions for the proposed deterministic model, and disease-free and endemic states have been identified. The threshold quantity known as the basic reproduction number \(\mathcal {R}_0\) has been determined using the next-generation matrix method. Subsequently, the local asymptotically stability (LAS) of the model has been analyzed using the linear stability theorem. Poincare-Bendixson theorem has been employed in the global asymptotically stability (GAS) analysis. The presence of a transcritical bifurcation has been established. Then, an NSFD scheme has been developed using Mickens’ approach. By examining the stability properties of the discretized model and comparing them with the corresponding continuous model, their dynamical consistency has been analyzed. Finally, some numerical simulations have been provided to illustrate our theoretical results.
1.2 The formulation of hepatitis B epidemic model
Let N(t) represent the entire population at time t, divided into three classes: susceptible individuals, infected individuals, and recovered individuals denoted respectively by S(t), I(t), and R(t). So, we have \(N(t)=S(t)+I(t)+R(t)\). Here we take into account a constant recruitment rate for the susceptible class, an incidence rate following the Beddington–DeAngelis type, vaccination of the susceptible class, and both natural and disease-induced death rates. Furthermore, we assume that certain infected individuals with sufficient physical strength can recover on their own without requiring medical treatment. Moreover, we make several assumptions for the model that are listed below:
- \(a_1.\):
-
The initial population sizes must be non-negative and denoted as S(0), I(0) and R(0).
- \(a_2.\):
-
All newborn individuals are initially assigned to the susceptible class.
- \(a_3.\):
-
The incidence rate is assumed to follow the Beddington–DeAngelis type.
- \(a_4.\):
-
Successfully vaccinated individuals will be moved to the recovered class.
- \(a_5.\):
-
The recovered population acquires permanent immunity.
By including all of the assumptions, the differential equation system of the HBV model can be formulated as follows:
with
Parameters used in HBV model (1.3) are non-negative.
1.3 The novelty of the paper
Most of the models examined in the literature are continuous epidemic models. However, as an important part of epidemiology, great importance is given to studies on epidemic models defined in a discrete structure. However, studies on discrete epidemic models are currently very few in the literature. Defining the model discretely or examining the continuous model by discretizing it has many advantages in epidemiology. The fact that epidemic data is generally collected in separate time units (such as daily, monthly or annual) is just one of them. Thus, using discrete models in the mathematical modeling of epidemic diseases may be more useful. These models are more advantageous than continuous ones. On the other hand, difference equations are discrete analogues of ordinary differential equations and are used to study their numerical solutions. In cases where analytical solutions of the system of differential equations cannot be obtained, its discrete structure can be used. Therefore, there is a need to discretize the system to calculate good analytical approximations of the solutions. Meanwhile, the resulting discrete model should preserve the dynamic properties of the original continuous model as much as possible. Recently, the nonstandard finite difference scheme was proposed by Mickens [30, 31] and has received much attention. An important advantage of the Mickens method is that it provides more effective protection of global asymptotic stability (compared to Euler and Runga Kutta methods). To our knowledge, there is no study in the literature that includes both vaccination and general incidence rates that examines both continuous and discrete models. Therefore, we consider a deterministic model that is more general than the models given in the literature and examine both its continuous structure and discrete structure.
1.4 Paper layout
The organization of this paper is as follows. In Sect. 2, we have investigated the dynamical properties of the HBV model (1.3), including the non-negativity and boundedness of solutions, the existence of equilibria, and the computation of the basic reproduction number. The local asymptotic stability of disease-free and endemic equilibria has been conducted. Lastly, the transcritical bifurcation behavior has been investigated. In Sect. 3, the NSFD scheme of the HBV model (1.3) has been constructed. Section 4 is dedicated to studying the dynamical properties of the discretized model that corresponds to the HBV model (1.3). The analysis of non-negativity, boundedness, and stability for the discretized model has been presented in this section. The obtained results indicate that there is dynamical consistency in terms of these properties between the discrete and continuous models. Finally, in the last section, we supplement our results with several numerical simulations for further clarification.
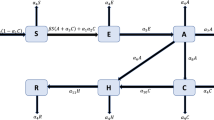
The schematic diagram of the HBV model (1.3) is shown in Fig. 1.
Flow diagram of the HBV model (1.3)
2 Dynamical analysis of the continuous model
In this section, we will analyze the qualitative behavior of the solutions of the HBV epidemic model (1.3) at the equilibrium points. First, we will examine the invariance interval and boundedness of solutions.
The requirement of non-negativity for S(t), I(t), and R(t), along with non-negative initial values for all \(t\ge 0\), is a fundamental condition in the HBV model (1.3) due to its biological nature. It is easy to see that the non-negative region \({\mathbb {R}}^3_+\) is a positively invariant set for the HBV model (1.3). Furthermore, it is essential to establish the boundedness of solutions in the HBV model as it reflects the constraints on available resources and prevents uncontrolled population growth. As such, ensuring the non-negativity and boundedness of solutions in the HBV model (1.3) is a biologically relevant consideration, which will be demonstrated in the following analysis. By summing the three equations in the HBV model (1.3) side by side, we reach the following population conservation law
By solving this ODE, we have
which implies that
where \(N(t)=S(t)+I(t)+R(t)\) denotes the total population size. Based on these results, it is enough to examine the HBV model (1.3) within the feasible region
Remark 1
As the first two equations in the HBV model (1.3) are independent of the third equation, it can be excluded without loss of generality. This leads to the reduction of the HBV model (1.3) as follows:
with
We will perform our analysis on the following feasible set:
2.1 Stability nature of the DFE
If we set the equations’ side on the right in the reduced HBV model (2.3) to zero and assume \(I=0\), it becomes immediately clear that the system always possesses a DFE point
Now, by determining the basic reproduction number denoted as \(\mathcal {R}_0\), locally asymptotically stability at the DFE point will be examined. We can find \(\mathcal {R}_0\) using the next-generation matrix method (see [35]). In the HBV model (2.3), there exists just one infected compartment, identified as I. The basic reproduction number is defined as the spectral radius of the next-generation matrix \(FV^{-1}\), where
Therefore, we have
Let us note that \(\mathcal {R}_0\) is the average number of secondary infections caused by a single infected individual in a population fully susceptible to the disease.
Theorem 1
The DFE point \(E_0\) of the HBV model (2.3) is LAS if \(\mathcal {R}_0<1\), and \(E_0\) is an unstable saddle point if \(\mathcal {R}_0>1\).
Proof
The Jacobian matrix of the HBV model (2.3) at the DFE point is
It is easy to obtain the eigenvalues of \(J_0\):
Clearly if \(\mathcal {R}_0<1\), then \(\lambda _{1,2}<0\). Hence the HBV model (2.3) is LAS at \(E_0\). Conversely, if \(\mathcal {R}_0>1\), then Jacobian matrix \(J_0\) has eigenvalues of both positive and negative signs. That is, \(\lambda _1\) is negative, while \(\lambda _2\) is positive. This indicates that the HBV model (2.3) is unstable, and \(E_0\) is also an unstable saddle point. Then, the proof is completed, as desired. \(\square \)
The result mentioned above indicates that when the basic reproduction number \(\mathcal {R}_0\) is less than 1, a small population of infected individuals will not be able to spread the infection. The spread of infection in this case depends on the initial size of the sub-populations.
Theorem 2
The DFE point \(E_0\) of the HBV model (2.3) is GAS if \(\mathcal {R}_0<1\).
Proof
If \(\mathcal {R}_0<1\), then there is no other equilibrium point than the disease-free equilibrium point (see (2.4)). Also, from Remark 1, positive solutions of the HBV model (2.3) are ultimately bounded and the S-axis is positively invariant, the I-axis repels the positive solutions. Since \(E_0\) is a locally asymptotically stable point, it follows from the Bendixson Theorem that every positive solution of the HBV model (2.3) approaches \(E_0\) as t approaches infinity. So, DFE point \(E_0\) of the HBV model (2.3) is globally asymptotically stable. The proof is completed, as desired. \(\square \)
2.2 Stability nature of the EE
To find the endemic equilibrium points, we have to set the differential equations of the HBV model (2.3) equal to zero. Then, we can obtain the endemic equilibrium \(E^*=(S^*,I^*)\), where
From the above equations, one can easily see that if \(\mathcal {R}_0>1\), then the EE point exists.
Theorem 3
The EE point \(E^*\) of the HBV model (2.3) is LAS if \(\mathcal {R}_0>1\).
Proof
Let’s assume \(\mathcal {R}_0>1\). After performing some algebraic calculations, one can easily find the Jacobian matrix evaluated at \(E^*\) as follows:
where
To have negative eigenvalues for the matrix mentioned above, both conditions must be satisfied: \(\text{ Tr }(J^*)<0\), and \(\det (J^*)>~0\) (see [26]). The trace of \(J^*\) can be easily obtained by
Also, the determinant of the matrix (2.5), after some algebraic calculations can be determined as follows:
Obviously if \(\mathcal {R}_0>1\), then the trace is negative while the determinant is positive for \(J^*\), which means that the EE point of the HBV model (2.3) is LAS. Thus, the proof is completed, as desired. \(\square \)
In the following theorem, we provide a necessary condition for the globally asymptotically stability of the endemic equilibrium point \(E^*\).
Theorem 4
If \(\mathcal {R}_0>1\), then the EE point \(E^*\) of the HBV model (2.3) is GAS in the interior of the positive quadrant \(\Omega \).
Proof
Let’s assume \(\mathcal {R}_0>1\). Consider the functions f(S, I) and g(S, I) as the functions right-hand side of the HBV model (2.3). Consider the Dulac function defined by
Then, we have
for all \((S,I)\in \Omega \). Hence the HBV model (2.3) has no periodic orbits in the \(\Omega \). Since all solutions of (2.3) are bounded and \(E_0\) is an unstable saddle point for \(\mathcal {R}_0>1\), from the Poincare-Bendixson theorem, we obtain the globally asymptotically stability of the endemic equilibrium \(E^*\). Thus, the proof is completed, as desired. \(\square \)
2.3 Transcritical bifurcation
The occurrence of a change in the stability behavior or dynamics of equilibrium points is commonly referred to as bifurcation (see [2, 36, 37]). An equilibrium point at which this change occurs is known as the bifurcation point. In this subsection, we will examine the behavior of the HBV model (2.3) when \(\mathcal {R}_0=1\).
Let \(S=\gamma _1\) and \(I=\gamma _2\) and denote by \(f_1(\gamma _1,\gamma _2)\) and \(f_2(\gamma _1,\gamma _2)\) the right-hand side functions of the HBV model (2.3). Then, the HBV model (2.3) can be rewritten as
The Jacobian matrix at DFE of the HBV model (2.3) evaluated at \(\mathcal {R}_0=1\) and \(\beta =\beta ^*\) where \(\beta ^*=\frac{(p+\mu +\alpha _1\Lambda )(\mu +\nu +\sigma )}{\Lambda }\) is given by
Indeed, the existence of a zero eigenvalue in matrix J indicates the existence of a bifurcation. Let the left and right eigenvectors corresponding to the zero eigenvalue of J be denoted as \(u=[u_1,u_2]^T\) and \(w=[w_1,w_2]\), respectively. One can easily calculate the components of u and w as follows:
From Theorem 4.1 in [37], the bifurcation constants can be computed as
Therefore, the HBV model (2.3) undergoes transcritical bifurcation at \(\mathcal {R}_0=1\). In other words, the stability of the DFE point transitions from stable to unstable when \(\mathcal {R}_0\) reaches a value of 1, indicating the existence of a positive equilibrium as \(\mathcal {R}_0\) crosses this threshold.
3 Constructing of the NSFD scheme
In this section, we will develop an NSFD scheme for the HBV model (1.3). Our aim is to formulate numerical approaches that preserve the essential features of the HBV model (1.3). These features include non-negativity of populations, population conservation law, and stability properties of DFE and EE points.
In the subsequent sections of this section, the variables \(S_n\), \(I_n\), and \(R_n\) will be used to denote the numerical approximations of S(t), I(t), and R(t), respectively, at discrete time points \(t=nh\), where \(n=0,1,2,\ldots \). Here, h represents the time-step size. To discretize the HBV model (1.3), we follow the approach proposed by Mickens (see [30]) in the following manner:
with
where \(\varphi (h)\) is the denominator function to be determined later. By adding all terms on both sides of the equations in the HBV model (3.1), we can obtain the expression:
which leads to the inequality:
where \(N_n=N(t_n)=S_n+I_n+R_n\). Notably, (3.3) serves as an approximation of the continuous population conservation law (2.1). To ensure the conservation of the population, we decide on the following formulation for the denominator function:
It is easy to see that substituting the denominator function (3.4) into the inequality (3.3) gives
Thus, by using mathematical induction, it is easy to see that for any \(h>0\), the solution of (3.3) satisfies the exact population conservation law (2.2):
Let us rewrite the HBV model (3.1) in the explicit form:
where
4 Dynamical analysis of the discrete HBV model
Here, we will explore the dynamical properties of the discrete HBV model (3.7). The results to be obtained here will demonstrate that the discrete HBV model (3.7) and the continuous HBV model (1.3) are dynamically consistent with the following properties:
-
Non-negativity of solutions,
-
Boundedness of solutions,
-
The existence of steady states,
-
Stability nature of equilibrium points.
Because of the non-negativity of all parameters in (3.7), and that \(\varphi (h)>0\) for all values of h, clearly \(S_{n+1},I_{n+1},R_{n+1}\ge 0\) if \(S_n,I_n,R_n\ge 0\). Therefore, the non-negative region \({\mathbb {R}}^3_+\) is a positively invariant set for the discrete HBV model (3.7). From (3.6), we have
These results imply that the discrete model is dynamically consistent with the continuous model concerning non-negativity and boundedness. Based on these result, we can conclude that a feasible region \(\Omega _2\) can be defined for analyzing the solutions of the HBV model (3.7) as follows;
Remark 2
By observing that the first two equations of the HBV model (3.7) are independent of \(R_n\), it is enough to only consider the first two equations without loss of generality. Thus, we can rewrite the HBV model (3.7) as
where \(S_n\ge 0\), \(I_n\ge 0\), \(\phi =\phi (S,I)=\frac{\beta I}{1+\alpha _1S+\alpha _2 I}\), \(\varphi =\varphi (h)\), and analyze it on the feasible set:
4.1 Stability nature of the DFE
We can verify that the discrete HBV model (4.1) and the continuous HBV model (2.3) share the same equilibrium points, namely, the DFE point \(E_0=(S_0,I_0)\) and the EE point \(E^*=(S^*,I^*)\). It should be noted that the EE point exists when the basic reproduction number \(\mathcal {R}_0\) is greater than 1.
Let us define the following functions for simplicity:
So, the Jacobian matrix of the discrete HBV model (4.1) at an equilibrium point \(E=(S,I)\) can be represented as:
To analyze the locally asymptotically stability of equilibrium points, we examine the magnitudes of eigenvalues of the Jacobian matrix evaluated at these points.
Theorem 5
If \(\mathcal {R}_0<1\), then the DFE point \(E_0\) of the discrete HBV model (4.1) is locally asymptotically stable regardless of the magnitude of h. Conversely, if \(\mathcal {R}_0>1\), then \(E_0\) is unstable regardless of the magnitude of h.
Proof
The Jacobian matrix of the HBV model (4.1) at \(E_0\) is
The eigenvalues of the matrix are
Obviously if \(\mathcal {R}_0<1\), then \(|\lambda _{1,2}|<1\) for all h. Therefore the discrete HBV model (4.1) is locally asymptotically stable at \(E_0\). Conversely, it is evident that if \(\mathcal {R}_0>1\), then \(\lambda _2>1\). This indicates that \(E_0\) is unstable. Thus, the proof is completed. \(\square \)
4.2 Stability nature of the EE
By conducting algebraic computations, we can establish that the discrete HBV model (4.1) shares the same positive equilibrium point as the continuous HBV model (2.3), that is the endemic equilibrium point as given in (2.4). This equilibrium point exists only if \(\mathcal {R}_0>1\).
Theorem 6
The EE point \(E^*\) of the discrete HBV model (4.1) is LAS if \(\mathcal {R}_0>1\), for all time-step sizes h.
Proof
Suppose that \(\mathcal {R}_0>1\). The Jacobian matrix of the HBV model (4.1) at EE is
where
and \(\phi ^*=\frac{\beta I^*}{1+\alpha _1S^*+\alpha _2I^*}\). From \(J(E^*)\), we have
and
Therefore, the EE point \(E^*\) is LAS if the condition \(|Tr(J)|<1+\det (J)<2\) is satisfied (see [26]). Hence, the proof is completed. \(\square \)
5 Numerical simulations and discussion
Simulations are used as important tools to assess the suitability of the proposed mathematical model for real-world scenarios. Here, some numerical simulations are provided to validate the theoretical results obtained in the paper. We will provide two examples for cases where \(\mathcal {R}_0\) is less and greater than 1. Through these examples, we will compare the Euler and RK4 schemes with the proposed NSFD scheme of the HBV model (1.3) and highlight the advantages of the NSFD approach. The simulations we will provide have been conducted using the MATLAB software.
Example 1
(\(\mathcal {R}_0<1\) Case) The parameters chosen for this example are provided in the Table 1. According to the parameters given in Table 1, it can be observed that
In Fig. 2, we simulate a comparison of numerical solutions of the HBV model (1.3) obtained by the Euler, RK4 and proposed NSFD scheme. We choose the initial values as \(S(0)=100\) and \(I(0)=40\) and time-step size \(h=4\). While Euler and RK4 schemes exhibit numerical inconsistencies such as unrealistic negative populations and failure to converge to the equilibrium point, the NSFD scheme does not suffer from these issues. In Fig. 3, it is demonstrated that the NSFD scheme provides consistent results for different time-step size values.
Example 2
(\(\mathcal {R}_0>1\) Case) Apart from the previous example, here \(\beta =2\), while all other parameters remain the same. In this case,
In Fig. 4, the global asymptotically stability of \(E^*\) can be observed from the \(S-I\) phase plane. There, the NSFD scheme is used for \(h=0.08\). Also, Fig. 5 illustrates the effect of vaccination rate p on susceptible and infected populations. Moreover, Figs. 6 and 7 show the effect of the parameters \(\alpha _1\) and \(\alpha _2\) on susceptible and infected populations, respectively.
6 Conclusions and remarks
In this study, we have investigated the dynamical properties of an HBV model with Beddington–DeAngelis type incidence and constant vaccination rate. We used the Beddington–DeAngelis type incidence rate, which includes both a measure of inhibition effect, such as preventive measures taken by susceptible individuals, and a measure of inhibition effect, such as treatment concerning infectives. This approach enabled us to study the HBV model (1.3), which yields results that are more meaningful and realistic when compared to the bilinear and saturated incidence rates. The non-negativity, boundedness, basic reproduction number, stability properties, and bifurcation of the HBV model (1.3) have been examined in detail. While locally asymptotically stability properties are demonstrated using the linear stability theorem, globally asymptotically stability of \(E^*\) has been shown using the Poincaré-Bendixson theorem. Stability conditions have been derived based on the threshold quantity \(\mathcal {R}_0\). The transcritical bifurcation has been proven using the center manifold theory.
Mathematical epidemic models are often expressed through systems of nonlinear differential or difference equations. It’s also worth noting that the model can be directly constructed using a system of difference equations. Given a model expressed in a system of differential equations, it is usually necessary to discretize it for practical purposes. In the literature, various standard numerical methods such as Euler or Runge–Kutta methods have been used to solve nonlinear differential equation systems. It has been demonstrated that these methods can potentially fail to preserve the dynamical properties of the corresponding continuous model. To avoid this dynamical inconsistency, discretized HBV model (3.1) has been derived by applying a non-locally approach to the non-linear terms for the continuous HBV model (1.3) and choosing a denominator function to satisfy the population conservation law. The discrete HBV model has been analyzed in terms of its non-negativity, boundedness, and stability properties. It has been concluded that both the discrete and continuous models maintain dynamical consistency for any finite step size. Finally, by presenting some numerical simulations, the theoretical results have been validated. Moreover, the provided simulations demonstrate that the parameters \(\alpha _1\), \(\alpha _2\) and p are significant factors in preventing the spread of the disease within the population.
In the near future, with the HBV model given by the system (1.3), further studies can be conducted on control strategies, rate of convergence, bifurcation analysis, local sensitivity analysis, and other related topics. Also, dynamically consistent NSFD schemes will be developed for several other epidemiological models to study their dynamical behavior.
Finally, let’s conclude the article by presenting a conjecture.
Conjecture
The DFE point \(E^0\) of the discrete HBV model (4.1) is GAS if \(\mathcal {R}_0<1\), and the EE point \(E^*\) is GAS if \(\mathcal {R}_0>1\), for all time-step sizes h.
Data availability
No data was used for the research described in the article.
References
Lavanchy, D., Kane, M.: Global Epidemiology of Hepatitis B Virus Infection. In: Liaw, YF., Zoulim, F. (eds) Hepatitis B Virus in Human Diseases. Molecular and Translational Medicine. Humana Press, Cham., 187–203 (2016). https://doi.org/10.1007/978-3-319-22330-8_9
Khan, T., Ullah, Z., Ali, N., Zaman, G.: Modeling and control of the hepatitis B virus spreading using an epidemic model. Chaos, Solitons Fractals 124, 1–9 (2019)
Hoang, M.T., Egbelowo, O.F.: On the global asymptotic stability of a hepatitis B epidemic model and its solutions by nonstandard numerical schemes. Bol. Sociedad Mat. Mex. 26(3), 1113–1134 (2020)
Cai, L., Zhaoqing, L., Xinyu, S.: Global analysis of an epidemic model with vaccination. J. Appl. Math. Comput. 57, 605–628 (2018)
Kulenovic, M.R.S., Nurkanovic, M., Yakubu, A.: Asymptotic behavior of a discrete-time density-dependent SI epidemic model with constant recruitment. J. Appl. Math. Comput. 67(1), 733–753 (2021)
Din, A.: The stochastic bifurcation analysis and stochastic delayed optimal control for epidemic model with general incidence function. Chaos Interdiscip. J. Nonlinear Sci. 31(12), 123101 (2021)
Din, A., Li, Y., Yusuf, A.: Delayed hepatitis B epidemic model with stochastic analysis. Chaos Solitons Fractals 146, 110839 (2021)
Din, A.: Bifurcation analysis of a delayed stochastic HBV epidemic model: cell-to-cell transmission. Chaos Solitons Fractals 181, 114714 (2024)
Khan, F.M., Khan, Z.U.: Numerical analysis of fractional order drinking mathematical model. J. Math. Tech. Model. 1(1), 11–24 (2024)
Khan, W.A., Zarin, R., Zeb, A., Khan, Y., Khan, A.: Navigating food allergy dynamics via a novel fractional mathematical model for antacid-induced allergies. J. Math. Tech. Model. 1(1), 25–51 (2024)
Ain, Q.T.: Nonlinear stochastic cholera epidemic model under the influence of noise. J. Math. Tech. Model. 1(1), 52–74 (2024)
Shah, S.M.A., Tahir, H., Khan, A., Arshad, A.: Stochastic model on the transmission of worms in wireless sensor network. J. Math. Tech. Model. 1(1), 75–88 (2024)
Cui, Q., Yang, X., Zhang, Q.: An NSFD scheme for a class of SIR epidemic models with vaccination and treatment. J. Differ. Equ. Appl. 20(3), 416–422 (2014)
Çakan, Ü.: Stability analysis of a mathematical model \(SI_uI_aQR\) for COVID-19 with the effect of contamination control (filiation) strategy. Fundam. J. Math. Appl. 4(2), 110–123 (2021)
Suryanto, A., Kusumawinahyu, W.M., Darti, I., Yanti, I.: Dynamically consistent discrete epidemic model with modified saturated incidence rate. Comput. Appl. Math. 32, 373–383 (2013)
Kermack, W.O., McKendrick, A.G.: A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. 115(772), 700–721 (1927)
Brauer, F., Castillo-Chavez, C.: Mathematical Models in Population Biology and Epidemiology, vol. 2. Springer, New York (2012)
Martcheva, M.: An Introduction to Mathematical Epidemiology, vol. 61. Springer, New York (2015)
Anderson, R.M., May, R.M.: Regulation and stability of host-parasite population interactions. J. Anim. Ecol. 47(1), 219–247 (1978)
Capasso, V., Serio, G.: A generalization of the Kermack–McKendrick deterministic epidemic model. Math. Biosci. 42(1–2), 43–61 (1978)
Beddington, J.: Mutual interference between parasites or predators and its effect on searching efficiency. J. Anim. Ecol. 44(1), 331–340 (1975)
DeAngelis, D.L., Goldstein, R.A., O’Neill, R.V.: A model for tropic interaction. Ecology 56(4), 881–892 (1975)
Kaddar, A.: On the dynamics of a delayed SIR epidemic model with a modified saturated incidence rate. Electron. J. Differ. Equ. 2009(133), 1–7 (2009)
Kaddar, A.: Stability analysis in a delayed SIR epidemic model with a saturated incidence rate. Nonlinear Anal. Model. Control 15(3), 299–306 (2010)
Dubey, B., Dubey, P., Dubey, U.S.: Dynamics of an SIR model with nonlinear incidence and treatment rate. Appl. Appl. Math. Int. J. 10(2), 718–737 (2016)
Allen, L.J.S.: Introduction to Mathematical Biology. Pearson/Prentice Hall, New Jersey (2007)
Yusuf, T.T., Benyah, F.: Optimal control of vaccination and treatment for an SIR epidemiological model. World J. Model. Simul. 8(3), 194–204 (2012)
Hu, Z., Teng, Z., Jiang, H.: Stability analysis in a class of discrete SIRS epidemic models. Nonlinear Anal. Real World Appl. 13(5), 2017–2033 (2012)
Darti, I., Suryanto, A.: Dynamics of a SIR epidemic model of childhood diseases with a saturated incidence rate continuous model and its nonstandard finite difference discretization. Mathematics 8(9), 1459 (2020)
Mickens, R.E.: Nonstandard Finite Difference Models of Differential Equations. World Scientific, Singapore (1994)
Mickens, R.E.: Nonstandard Finite Difference Schemes: Methodology and Applications. World Scientific, New Jersey (2020)
Suryanto, A.: A dynamically consistent nonstandard numerical scheme for epidemic model with saturated incidence rate. Int. J. Math. Comput. 13, 112–123 (2011)
Suryanto, A., Darti, I.: On the nonstandard numerical discretization of SIR epidemic model with a saturated incidence rate and vaccination. AIMS Math. 6, 141–155 (2021)
Ding, D., Ma, Q., Ding, X.: A non-standard finite difference scheme for an epidemic model with vaccination. J. Differ. Equ. Appl. 19(2), 179–190 (2013)
Van den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180(1–2), 29–48 (2022)
Aloqeili, M., Shareef, A.: Neimark–Sacker bifurcation of a third order difference equation. Fundam. J. Math. Appl. 2(1), 40–49 (2019)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1(2), 361–404 (2004)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gümüş, M., Türk, K. Dynamical behavior of a hepatitis B epidemic model and its NSFD scheme. J. Appl. Math. Comput. (2024). https://doi.org/10.1007/s12190-024-02103-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12190-024-02103-6