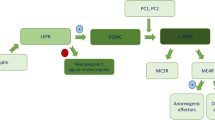
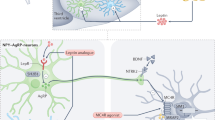
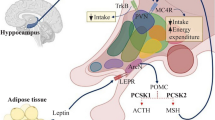
Abstract
Purpose of Review
The prevalence of pediatric obesity has increased significantly over the past couple of generations. While monogenic obesity, syndromic obesity, and endocrinopathies associated with obesity have been increasingly recognized, they do not account for the increase in prevalence. We describe these rare conditions and the dysregulation of neuropathways in obesity and review successes and failures in treatments in both syndromic and nonsyndromic obesity.
Recent Findings
The best-described form of syndromic obesity is Prader–Willi Syndrome (PWS). While recent pharmacotherapies (specifically beloranib) demonstrated improvements in weight in PWS, the unfortunate adverse effect of deep vein thrombosis and pulmonary embolism necessitated the halting of its further development. Additional treatments are in development which target the signaling of ghrelin and other hypothalamic targets known to be dysregulated in PWS. For nonsyndromic obesity, lifestyle modifications remain the mainstay of treatment. However, recent large-scale interventions have had disappointing results. Bariatric surgery in children holds some promise, though complications and reoperations are common. Pharmacotherapies have been developed that treat rare monogenic forms of obesity, including MC4R agonists, which hold promise for these uncommon explanations for early childhood weight gain. There is evidence that methylation patterns in key genes in the neuroregulation of appetite are altered in individuals with obesity. Interestingly, this altered methylation is evident in sperm, which may have an impact on the heritability of gene expression across generations.
Summary
Pediatric obesity is complex and multifactorial. Efforts in rare monogenic and syndromic obesity may give rise to potential treatment opportunities in circumstances where lifestyle interventions are unsuccessful.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Farooqi IS, Keogh JM, Yeo GSH, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. https://doi.org/10.1056/NEJMoa022050.
Farooqi IS, Yeo GSH, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–9. https://doi.org/10.1172/JCI9397.
Collet T-H, Dubern B, Mokrosinski J, et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol Metab. 2017;6(10):1321–9. https://doi.org/10.1016/j.molmet.2017.06.015.
Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237–47. https://doi.org/10.1056/NEJMoa063988.
Stijnen P, Ramos-Molina B, O’Rahilly S, Creemers JWM. PCSK1 mutations and human endocrinopathies: from obesity to gastrointestinal disorders. Endocr Rev. 2016;37(4):347–71. https://doi.org/10.1210/er.2015-1117.
Thaker VV. Genetic and epigenetic causes of obesity. Adolesc Med State Art Rev. 2017;28(2):379–405 http://www.ncbi.nlm.nih.gov/pubmed/30416642. Accessed July 6, 2019.
Crujeiras AB, Campion J, Díaz-Lagares A, et al. Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul Pept. 2013;186:1–6. https://doi.org/10.1016/j.regpep.2013.06.012.
Barrès R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–11. https://doi.org/10.1016/j.cmet.2012.01.001.
Lindholm ME, Marabita F, Gomez-Cabrero D, et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics. 2014;9(12):1557–69. https://doi.org/10.4161/15592294.2014.982445.
Donkin I, Versteyhe S, Ingerslev LR, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23(2):369–78. https://doi.org/10.1016/j.cmet.2015.11.004.
de Castro BT, Ingerslev LR, Alm PS, et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab. 2016;5(3):184–97. https://doi.org/10.1016/j.molmet.2015.12.002.
Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Genet. 2019;95(1):23–40. https://doi.org/10.1111/cge.13367.
Chung WK. An overview of monogenic and syndromic obesities in humans. Pediatr Blood Cancer. 2012;58(1):122–8. https://doi.org/10.1002/pbc.23372.
D’Angelo CS, Varela MC, de Castro CIE, et al. Chromosomal microarray analysis in the genetic evaluation of 279 patients with syndromic obesity. Mol Cytogenet. 2018;11(1):14. https://doi.org/10.1186/s13039-018-0363-7.
Elena G, Bruna C, Benedetta M, Stefania DC, Giuseppe C. Prader-Willi syndrome: clinical aspects. J Obes. 2012;2012:1–13. https://doi.org/10.1155/2012/473941.
Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Investig. 2015;38(12):1249–63. https://doi.org/10.1007/s40618-015-0312-9.
Buiting K. Prader-Willi syndrome and Angelman syndrome. Am J Med Genet C Semin Med Genet. 2010;154C(3):365–76. https://doi.org/10.1002/ajmg.c.30273.
Crinò A, Fintini D, Bocchini S, Grugni G. Obesity management in Prader–Willi syndrome: current perspectives. Diabetes, Metab Syndr Obes Targets Ther. 2018;11:579–93. https://doi.org/10.2147/DMSO.S141352.
Emerick JE, Vogt KS. Endocrine manifestations and management of Prader-Willi syndrome. Int J Pediatr Endocrinol. 2013;2013(1):14. https://doi.org/10.1186/1687-9856-2013-14.
Burman P, Ritzén EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev. 2001;22(6):787–99. https://doi.org/10.1210/edrv.22.6.0447.
Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. 2012;59(10):889–94 http://www.ncbi.nlm.nih.gov/pubmed/22785236. Accessed July 6, 2019.
De Waele K, Ishkanian SL, Bogarin R, et al. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader-Willi syndrome. Eur J Endocrinol. 2008;159(4):381–8. https://doi.org/10.1530/EJE-08-0462.
McCandless SE, Yanovski JA, Miller J, et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader–Willi syndrome: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;19(12):1751–61. https://doi.org/10.1111/dom.13021.
Kuppens RJ, Donze SH, Hokken-Koelega ACS. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin Endocrinol. 2016;85(6):979–87. https://doi.org/10.1111/cen.13169.
Alsaif M, Elliot SA, MacKenzie ML, Prado CM, Field CJ, Haqq AM. Energy metabolism profile in individuals with Prader-Willi syndrome and implications for clinical management: a systematic review. Adv Nutr An Int Rev J. 2017;8(6):905–15. https://doi.org/10.3945/an.117.016253.
Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2008;46(1):80–3. https://doi.org/10.1097/01.mpg.0000304458.30294.31.
Irizarry KA, Miller M, Freemark M, Haqq AM. Prader Willi Syndrome: genetics, metabolomics, hormonal function, and new approaches to therapy. Adv Pediatr Infect Dis. 2016;63(1):47–77. https://doi.org/10.1016/j.yapd.2016.04.005.
Forsythe E, Beales PL. Bardet-Biedl Syndrome.; 1993. http://www.ncbi.nlm.nih.gov/pubmed/20301537. Accessed July 6, 2019.
Khan SA, Muhammad N, Khan MA, Kamal A, Rehman ZU, Khan S. Genetics of human Bardet-Biedl syndrome, an updates. Clin Genet. 2016;90(1):3–15. https://doi.org/10.1111/cge.12737.
Suspitsin EN, Imyanitov EN. Bardet-Biedl Syndrome. Mol Syndromol. 2016;7(2):62–71. https://doi.org/10.1159/000445491.
Daniels AB, Sandberg MA, Chen J, Weigel-DiFranco C, Fielding Hejtmancic J, Berson EL. Genotype-phenotype correlations in Bardet-Biedl syndrome. Arch Ophthalmol (Chicago, Ill 1960). 2012;130(7):901–7. https://doi.org/10.1001/archophthalmol.2012.89.
Dervisoglu E, Isgoren S, Kasgari D, Demir H, Yilmaz A. Obesity control and low protein diet preserve or even improve renal functions in Bardet-Biedl syndrome: a report of two cases. Med Sci Monit. 2011;17(1):CS12–4. https://doi.org/10.12659/MSM.881320.
Álvarez-Satta M, Castro-Sánchez S, Valverde D. Alström syndrome: current perspectives. Appl Clin Genet. 2015;8:171–9. https://doi.org/10.2147/TACG.S56612.
Marshall JD, Bronson RT, Collin GB, et al. New Alström Syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165(6):675. https://doi.org/10.1001/archinte.165.6.675.
Kelly J. Alstrom syndrome; ALMS. https://omim.org/entry/203800. Published 2016. Accessed March 1, 2019.
Girard D, Petrovsky N. Alström syndrome: insights into the pathogenesis of metabolic disorders. Nat Rev Endocrinol. 2011;7(2):77–88. https://doi.org/10.1038/nrendo.2010.210.
Lodh S, Hostelley TL, Leitch CC, O’Hare EA, Zaghloul NA. Differential effects on β-cell mass by disruption of Bardet–Biedl syndrome or Alstrom syndrome genes. Hum Mol Genet. 2016;25(1):57–68. https://doi.org/10.1093/hmg/ddv447.
Tai T-S, Lin S-Y, Sheu WH-H. Metabolic effects of growth hormone therapy in an Alström syndrome patient. Horm Res. 2003;60(6):297–301. https://doi.org/10.1159/000074248.
Sinha SK, Bhangoo A, Anhalt H, et al. Effect of metformin and rosiglitazone in a prepubertal boy with Alström syndrome. J Pediatr Endocrinol Metab. 2007;20(9):1045–52 http://www.ncbi.nlm.nih.gov/pubmed/18038714. Accessed July 6, 2019.
Zufferey F, Sherr EH, Beckmann ND, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet. 2012;49(10):660–8. https://doi.org/10.1136/jmedgenet-2012-101203.
Bochukova EG, Huang N, Keogh J, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463(7281):666–70. https://doi.org/10.1038/nature08689.
D’Angelo CS, Koiffmann CP. Copy number variants in obesity-related syndromes: review and perspectives on novel molecular approaches. J Obes. 2012;2012:1–15. https://doi.org/10.1155/2012/845480.
Tiberio G, Digilio MC, Giannotti A. Obesity and WAGR syndrome. Clin Dysmorphol. 2000;9(1):63–4 http://www.ncbi.nlm.nih.gov/pubmed/10649802. Accessed July 6, 2019.
Han JC. Rare syndromes and common variants of the brain-derived neurotrophic factor gene in human obesity. In: Progress in Molecular Biology and Translational Science. Vol 140. ; 2016:75-95. doi:https://doi.org/10.1016/bs.pmbts.2015.12.002.
Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinol Metab Clin N Am. 2009;38(3):525–48. https://doi.org/10.1016/j.ecl.2009.06.007.
Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity—assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–57. https://doi.org/10.1210/jc.2016-2573.
Lodish MB, Keil MF, Stratakis CA. Cushing’s syndrome in pediatrics. Endocrinol Metab Clin N Am. 2018;47(2):451–62. https://doi.org/10.1016/j.ecl.2018.02.008.
Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin N Am. 2012;41(4):793–803. https://doi.org/10.1016/j.ecl.2012.08.002.
Magiakou MA, Mastorakos G, Oldfield EH, et al. Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med. 1994;331(10):629–36. https://doi.org/10.1056/NEJM199409083311002.
Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40. https://doi.org/10.1210/jc.2008-0125.
Quattrin T, Wilfley DE. The promise and opportunities for screening and treating childhood obesity. JAMA Pediatr. 2017;171(8):733. https://doi.org/10.1001/jamapediatrics.2017.1604.
O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents. JAMA. 2017;317(23):2427. https://doi.org/10.1001/jama.2017.0332.
Wilfley DE, Saelens BE, Stein RI, et al. Dose, content, and mediators of family-based treatment for childhood obesity: a multisite randomized clinical trial. JAMA Pediatr. 2017;171(12):1151–9. https://doi.org/10.1001/jamapediatrics.2017.2960.
Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in overweight children? J Clin Endocrinol Metab. 2016;101(8):3171–9. https://doi.org/10.1210/jc.2016-1885.
Wilfley DE, Hayes JF, Balantekin KN, Van Buren DJ, Epstein LH. Behavioral interventions for obesity in children and adults: evidence base, novel approaches, and translation into practice. Am Psychol. 2018;73(8):981–93. https://doi.org/10.1037/amp0000293.
Dietz WH. We need a new approach to prevent obesity in low-income minority populations. Pediatrics. 2019;143(6):e20190839. https://doi.org/10.1542/peds.2019-0839.
Moore SM, Borawski EA, Love TE, et al. Two family interventions to reduce BMI in low-income urban youth: a randomized trial. Pediatrics. 2019;143(6):e20182185. https://doi.org/10.1542/peds.2018-2185.
Barkin SL, Heerman WJ, Sommer EC, et al. Effect of a behavioral intervention for underserved preschool-age children on change in body mass index: a randomized clinical trial. JAMA. 2018;320(5):450–60. https://doi.org/10.1001/jama.2018.9128.
French SA, Sherwood NE, Veblen-Mortenson S, et al. Multicomponent obesity prevention intervention in low-income preschoolers: primary and subgroup analyses of the NET-works randomized clinical trial, 2012-2017. Am J Public Health. 2018;108(12):1695–706. https://doi.org/10.2105/AJPH.2018.304696.
Pilitsi E, Farr OM, Polyzos SA, et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019;92:170–92. https://doi.org/10.1016/j.metabol.2018.10.010.
Ryder JR, Fox CK, Kelly AS. Treatment options for severe obesity in the pediatric population: current limitations and future opportunities. Obesity (Silver Spring). 2018;26(6):951–60. https://doi.org/10.1002/oby.22196.
Sherafat-Kazemzadeh R, Yanovski SZ, Yanovski JA. Pharmacotherapy for childhood obesity: present and future prospects. Int J Obes. 2013;37(1):1–15. https://doi.org/10.1038/ijo.2012.144.
Srivastava G, Fox CK, Kelly AS, et al. Clinical considerations regarding the use of obesity pharmacotherapy in adolescents with obesity. Obesity (Silver Spring). 2019;27(2):190–204. https://doi.org/10.1002/oby.22385.
Inge TH, Courcoulas AP, Jenkins TM, et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019;380(22):2136–45. https://doi.org/10.1056/NEJMoa1813909.
Beets MW, Brazendale K, Weaver RG, Armstrong B. Rethinking behavioral approaches to compliment biological advances to understand the etiology, prevention, and treatment of childhood obesity. Child Obes 2019:chi.2019.0109. doi:https://doi.org/10.1089/chi.2019.0109.
Fox CK, Kelly AS. The potential role of combination pharmacotherapy to improve outcomes of pediatric obesity: a case report and discussion. Front Pediatr. 2018;6. https://doi.org/10.3389/fped.2018.00361.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shashikala Gowda and Tasa Seibert declare that they have no conflict of interest.
Ryan Farrell has been principal investigator and/or coinvestigator for three different industry-sponsored pharmaceutical trials for Prader–Willi Syndrome (GLWL, Soleno, and Levo Therapeutics).
Naveen Uli has been principal investigator and/or coinvestigator for three different industry-sponsored pharmaceutical trials for Prader–Willi Syndrome (GLWL, Soleno, and Levo Therapeutics). He also was a coinvestigator for the Childhood Obesity Prevention and Treatment Research Consortium (COPTR).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Obesity and Diet
Rights and permissions
About this article
Cite this article
Gowda, S., Seibert, T., Uli, N. et al. Pediatric Obesity: Endocrinologic and Genetic Etiologies and Management. Curr Cardiovasc Risk Rep 13, 39 (2019). https://doi.org/10.1007/s12170-019-0632-y
Published:
DOI: https://doi.org/10.1007/s12170-019-0632-y