Abstract
In this study, Fourier transform mid-infrared (FT-MIR) spectroscopy was investigated as a rapid and non-destructive method for the determination of selected quality parameters of oat flakes and cakes. The spectra of 14 commercial products were registered in the spectral range of 4,000–370 cm−1. Chemometric approach was applied to collect spectral and reference data on fat content, fatty acid composition, and the heat of combustion, and to correlate them. Reference data were obtained with the use of Soxhlet method, gas chromatography, and bomb calorimetry. Statistically significant coefficients in the determination of spectral data and fat, linoleic acid, stearic acid, palmitic acid content, and energy value are R 2 = 0.93, R 2 = 0.93, R 2 = 0.82, R 2 = 0.92, and R 2 = 0.95, respectively. The energy value, as well as the saturated and monounsaturated fatty acid content, was statistically higher in cakes as compared to that in flakes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cakes available in the market differ in composition due to a variety of ingredients applied by producers. Some of commercial products contain natural additives, e.g., milk, flakes, honey, or dried fruits that increase nutritional value, improve taste, and regulate flavor. Due to the emphasis of dietary specialists on the need to change eating habits of the population towards healthier lifestyle, many consumers pay more attention to health-quality parameters of food, such as fat content, fatty acid composition, or caloric value. For safety reasons, monitoring and control bodies require rapid and robust analysis of different foodstuffs available in the market, including cakes, and evaluation of producer-labeled declarations. To achieve this aim, sophisticated analytical techniques are needed to be applied. One of them is Fourier transform infrared spectroscopy (FT-IR) which is a very useful technique widely applied to determine the content and structure of chemical compounds in variety of foodstuffs. Due to its widely presented advantages (Bureau et al. 2009; Che Man and Setiowaty 1999; Kim et al. 2007; Maggio et al. 2009; Moros et al. 2005; Rohman et al. 2011; Silva et al. 2009; Syahariza et al. 2005; Vlachos et al. 2006), FT-IR method is more often used for the assessment of the quality of food. The application of FT-IR substantially decreases the analysis time, makes sample preparation a lot easier, and requires less amount of chemical solvents compared to standard reference methods (such as Soxhlet method or gas chromatography). Studies done in recent years prove that FT-IR technique is very useful for the determination of the number of quality parameters of edible oils (Che Man and Setiowaty 1999; Maggio et al. 2009; Silva et al. 2009; Bryś et al. 2013), drinks (Moros et al. 2005; Arzberger and Lachenmeier 2008; Bauer et al. 2008), fruits (Bureau et al. 2009; Kim et al. 2009), vegetables (Beullens et al. 2006), meat (Rohman et al. 2011), and others (Galvis-Sánchez et al. 2013; Ostrowska-Ligęza et al. 2012).
Infrared spectroscopy is commonly used for the quality assessment of raw materials, e.g., cereal grains, but it is also possible for the analysis of commercial cereal products, e.g., cakes. Most studies conducted in the last decade often refer to near-infrared region rather than mid-infrared (Berardo et al. 2004; Pettersson and Aberg 2003; Wang et al. 2006; Kim and Kays 2009; Kays et al. 2005; Kays and Barton 2002). Kim and Kays (Kim and Kays 2009), Kays et al. (Kays et al. 2005), and Kays and Barton (Kays and Barton 2002) verified that NIR spectroscopy is a good and accurate tool in determining fat and trans fatty acid content and the heat of combustion of various cereal products. A few publications only refer to MIR region, for example, Fourier transform mid-infrared spectroscopy was used by Kim et al. (Kim et al. 2007) to determine the content of trans fatty acids in cereal products available in the market. The results of their work indicate that the said method can easily replace time- and labor-consuming gas chromatography or other methods. FT-MIR spectroscopy is also successfully applied for the determination of lard adulteration in cakes (Syahariza et al. 2005).
The main difference between MIR and NIR is the energy absorbed by vibrating molecules. Absorption in MIR corresponds to the fundamental bands of molecular vibrations, whereas NIR spectrum consists of weak bands corresponding to overtones and combination absorptions. The advantage of NIR over MIR is that the NIR light can penetrate much farther into a sample than MIR radiation. This allows the measurement of the samples in near infrared transmitting, packaging materials (for example glass or plastic) and requires less or no sample preparation. On the other hand, MIR absorption bands are much more specific and easier to assign which allow more accurate qualitative analysis of the components in levels lower than 1 % (van de Voort et al. 2001; Dufour 2008; Lin et al. 2008).
The objective of this study was to assess the application of FT-MIR method in the evaluation of the fat content, fatty acid composition, and caloric value of organic oat flakes and cakes available in the market. Statistical models based on PLS technique and classical Beer–Lambert law were worked out and evaluated.
Materials and Methods
Samples
Fourteen certified organic oat products were purchased from local shops. The products included eight brands of oat flakes (A1–A8) and six types of oat cakes (C1 cocoa oat cakes-brand 1, C2 raisin oat cakes-brand 1, C3 cocoa oat cakes-brand 2, C4 oatmeal nut cakes-brand 2, C5 cinnamon raisin oat cakes-brand 2, and C6 organic oat cakes with honey-brand 3).
Fat Content
Fat content was determined by Soxhlet extraction method using petroleum ether as solvent. Extractions were carried out using semiautomatic Soxtec Avanti 2055 System (FOSS, Hillerød, Denmark) at 155 °C for 120 min. The moisture content of the samples was determined using the oven drying method at 105 °C for 4 h.
Fatty Acid Composition
The samples were ground and extracted with hexane by shaking for 30 min. Then, the mixture was filtered, and the fat was obtained by evaporating the solvent from the filtrate. Fat extracted from the studied samples was esterified with methanol. One drop of oil (0.05 ml) was mixed with 2 cm3 of hexane, then 2 cm3 of the solution of CH3OK in methanol (1 mol/dm3) was added. The mixture was kept in 40 °C and was mixed throughout the whole reaction. After the reaction was completed, upper phase/layer was collected for gas chromatographic measurement.
Gas chromatography analysis was performed using GC–17A Shimadzu (Shimadzu, Japan) apparatus equipped with flame ionization detector and capillary column with 30-m length, 0.22-mm i.d. and 0.25-μm film thickness. The following temperature programming was used: 60 °C for 1 min, then it was increased by 10 °C min−1 to 170 °C; from 170 to 230 °C, it was increased by 3 °C min−1, then kept at 230 °C for 15 min. The temperature of the split injector was 225 °C, with a split ratio of 1:100; the detector temperature was 250 °C. Nitrogen flowing with the rate of 1 ml/min was used as the carrier gas. The identification of fatty acids was carried out using the lipid standard purchased from Sigma–Aldrich.
Calorific Value
The heat of combustion was determined using bomb calorimetry method (Smit et al. 2004; Miller and Payne 1959). Sample preparation involved grinding the sample and creating pellet with a weight of about 1 g. Analysis was performed using KL-12Mn (PRECYZJA-BIT, Poland) bomb calorimeter. Heat of combustion of a sample was obtained based on the changes in temperature of water surrounding the bomb and exact weight of the sample. The result was automatically calculated by a calorimeter software.
FT-IR Analysis
The infrared spectra of intact samples were recorded with the aid of Perkin–Elmer system 2000 spectrometer (Perkin–Elmer, USA). The apparatus was entirely software-controlled by GRAMS Research software. The spectra were registered in the spectral range of 4,000–370 cm−1. Samples mixed with potassium bromide (background) were analyzed in the form of thin pellets. Firstly, the spectrum of the pure background (potassium bromide) was recorded, then the spectra of the samples mixed with potassium bromide. The spectra of the samples were obtained in three replicates. The spectra were processed with PEGRAMS software; baseline and offset corrections were applied.
Statistical Analysis
One-way ANOVA technique was applied to evaluate the fat content and energy value of the selected products. It was achieved with the use of Statistica 6.0 software. Simple regression models (calibration based on single band intensity and calibration based on intensity ratios of bands) were created with the aid of Statistica 6.0 software. PLS models were developed with TQ Analyst software.
Results and Discussion
Reference Analysis
Fat Content and the Energy Value
The heat of combustion and fat content values of the studied cakes are presented in Table 1. The fat content of the oat flakes ranged from 6.0 to 7.3 %. Rzedzicki (Rzedzicki 2006) reported wider range of fat content in oat flakes (6.3–10.1 %). The oat cakes were characterized with higher and more diversified fat content (8.9–29.6 %). Statistically significant differences (p < 0.05 at confidence interval 95 %) were observed for four analyzed cakes (C1, C2, C3, and C6). C6 sample was characterized with the lowest fat content among the oat cakes (8.9 %) due to fat-free processing of this product, as the producer claims. The content of fat in cakes depends on the amount of fat specified in the formula. It can also be affected by some natural additives like nuts or cocoa.
The energy value was expressed as joules of energy released per 1 g of sample (heat of combustion). All oat flakes were characterized by similar heat of combustion (17.6–18.5 kJ/g, p > 0.05 at confidence interval 95 %); however, cakes had higher energy value as shown in Table 1.
Fatty Acid Composition
Several fatty acids were indentified in analyzed oat products. The percentage of fatty acids is presented in Table 2. Lipid fraction of the majority of the oat cakes was of higher content of saturated fatty acids (SFA) than fat extracted from flakes. For five studied cakes, the content of SFA in lipid fraction ranged from 38.3 to 46.4 %, whereas for flakes, the values ranged from 17.5 to 24.8 %. The lowest value of that parameter, compared to all of the studied products, was noted in cakes C6 (8.9 %).
The content of monounsaturated fatty acids (MUFA) in lipid fraction was similar in almost all cakes and flakes (36.7–46.0 %), with the exception of product C6 which was with higher value of monounsaturated fatty acid (71.2 % of lipid fraction).
The content of polyunsaturated fatty acids (PUFA) in lipid fraction of flakes was around 31.3–45,8 %. Fat extracted from all studied cakes had lower content of PUFA; the highest value (20.4 %) was noted in C4, and the lowest (11.5 %) in C1.
Two essential fatty acids, namely, linoleic and linolenic acid, were identified in all studied products. The content of linoleic acid in fat extracted from flakes and cakes was 30.5–44.4 % and 10.7–20.2 %, respectively. Linolenic acid occurred in much smaller quantities; the content of this compound in the lipid fraction of each product did not exceed 2.0 %.
FT-IR Analysis
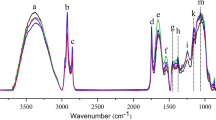
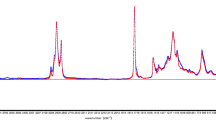
The spectra of oat cakes and flakes/cereal products contain several characteristic spectral bands generated by vibrations of molecules of compounds present in the sample, e.g. fats, carbohydrates or proteins. Figure 1 shows the spectra of oat cake and flake selected from the data set, with marked spectral regions that indicate various functional groups (van de Voort et al. 2001; Dufour 2008; Lin et al. 2008).
Calibration Models
Fifteen spectral ranges were selected manually for calibration; the selection was based on visible differences between the spectra of the studied products followed by numerical subtraction to prove differences. In selected regions, the differences were statistically significant (p < 0.05) (Fig. 1, Table 3). Intensities of single bands as well as ratios of intensities of those bands were used to create calibration models of fat content, fatty acid composition, and energy value (Figs. 2 and 3). Areas underneath the selected bands were calculated manually with the aid of Pegrams 2000 software; the ratios of the intensities of spectral bands were equated with Excel 2007 software.
The samples of the oat flakes and cakes were divided into calibration and validation sets; the values of the samples in each set are presented in Tables 4, 5 and 6. Cross-validation process was used in creating every calibration model.
Calibration Models Based on the Intensity of Single Band
The best correlations between analyzed quality parameters and spectral data are presented in Table 4. Relatively high correlation was found for fat content, R 2 = 0.68. However, high values of standard error of cross-validation (SECV) and standard error of prediction (SEP) indicate that the created model cannot be used for the analysis of fat content.
Calibration model created for energy value was characterized with high values of determination coefficient (R 2 = 0.84) and relatively low values of SECV (3.7 %) and SEP (6.7 %). Calibration models were created for the content of five fatty acids (eicosenoic acid, linoleic acid, linolenic acid, myristic acid, oleic acid, and stearic acid). Relatively high correlation was found only for linoleic acid (R 2 = 0.84), however, high values of SECV and SEP (Table 4) indicate low applicability of the model.
Calibration Models Based on the Intensity Ratios of Two Bands
The application of intensities of two spectral bands for calibration models produced better statistical results in the case of fat content, energy value, and linoleic acid. Higher values of coefficients of determination were observed. Also, SECV and SEP values were lower as compared to single band intensity calibration models (Tables 4 and 5).
The best model was created for energy value, with R 2 = 0.95 and very low values of SECV and SEP, both equaled to 2 %. This calibration was done with the use of intensity ratio I13/I14 (nomenclature of abbreviations of spectral bands is presented in Table 3). According to Guillen and Cabo (Guillen and Cabo 1997), the spectral ranges 800–749 and 738–697 cm−1 can be attributed to overlapping ―(CH2) n ― rocking vibration and out-of-plane ―CH═CH― (cis) bending vibration of fatty acids.
Good correlation was found between spectral data and the content of palmitic acid, with a coefficient of determination of 0.92. High values of SECV and SEP indicated, however, that the found relation cannot be used for the evaluation of this quality parameter. Relatively good correlation was observed for stearic acid content with coefficient of determination equal to 0.67.
PLS Modeling
In order to create robust PLS models, statistical spectra diagnostic was run for all quality parameters investigated in this study. Statistical spectra illustrate the spectral regions that correlate in the highest degree with changes of value of quality parameter of analyzed samples. Spectral ranges selected manually in this research (Table 3) were evaluated through this test. The results of the statistical spectra diagnostic are presented in Table 7.
The number of factors in each model was established based on the predicted residual error sum of squares and standard error of cross-validation. Graphic interpretation of each model (Fig. 4) indicated good correlation between spectral data and fat content, energy value, palmitic acid content, and linoleic acid content. Statistical results of the calibration models with highest correlations are presented in Table 7.
Compared to the models previously described in this paper, statistically significant coefficients of determination were observed for all analyzed quality parameters, but they did not exceed the values presented for the calibration based on intensity ratios of two bands. The only improvement in the statistical parameters was observed in the palmitic acid content (the decrease in SECV and SEP values).
The best calibration was noted for energy value with high R 2 and low SECV and SEP values (Table 6). This statistical model was developed based on the data from spectral regions I1, I2, I4, I5, and I6. Spectral band in the range 2,990–2,885 cm−1 (I1) is attributed to the stretching vibrations of C―H bond in the aliphatic chain of fatty acids. In spectral region 1,770–1,725 cm−1 (I2) the absorption of ―C═O stretching vibration occurs. The bands in spectral regions 1,479–1,453 cm−1 (I4) and 1,426–1,409 cm−1 (I5) are linked with ―CH2― scissoring and ―CH═(cis) bending. The last band used in this calibration (I6) can be attributed to the scissoring vibration of group ―CH3 (van de Voort et al. 2001; Guillen and Cabo 1997).
Conclusion
The actual values of fat content, fatty acid composition, and energy value of selected organic oat products were obtained by conventional methods. Reference data of investigated quality parameters were used to create FT-MIR calibration models. Although statistically significant correlations were observed for all quality parameters, the relations found for fat content and fatty acid composition are not suitable for accurate analysis. It is indicated by low values of SECV (fat content 17.5–38.5 %, fatty acid composition 11.4–29.7 %) and SEP (fat content 20.9–38.4 %, fatty acids composition 21.5–53.2 %). Substantial increase of the number of samples in calibration and validation data set might result in the improvement of parameters for statistical models.
The most robust model was found for energy value with the determination coefficient equal to 0.95 (SECV = 2.1 %, SEP = 2.0 %) when intensity ratios of bands were used for calibration. When, instead of simple Lambert–Beer law, the intensities of the two spectral bands were used for calibration, vast improvement in statistical parameters was observed. Statistical results of models developed with PLS software were comparable with those obtained for intensity ratios of bands.
Statistical testing indicated that intensity ratios of bands and PLS calibration models created for energy value might be suitable for analytical use. Replacing conventional bomb calorimetry method with FT-IR method would result in considerable decrease of time analysis from 40 min to few seconds without changes in accuracy.
References
Arzberger U, Lachenmeier DW (2008) Food Anal Methods 1:18
Bauer R, Nieuwoudt H, Bauer FF, Kossmann J, Koch KR, Esbensen KH (2008) Anal Chem 80:1371
Berardo N, Brenna OV, Amato A, Valoti P, Pisacane V, Motto M (2004) Innovative Food Sci Emerg Technol 5:393
Beullens K, Dmitriy Kirsanov D, Joseph Irudayaraj J, Alisa Rudnitskaya A, Andrey Legin L, Bart M, Nicola BM, Lammertyna J (2006) Sens Actuators B 116:107
Bryś J, Wirkowska M, Górska A, Ostrowska-Ligenza E, Bryś A, Koczoń P (2013) J Therm Anal Calorim 112:481
Bureau S, Ruiz D, Reich M, Gouble B, Bertrand D, Audergon J-M, Catherine MGC, Renard CMGC (2009) Food Chem 115:1133
Che Man YB, Setiowaty G (1999) Food Chem 67:193
Dufour É (2008) In: Sun DW (ed) Infrared spectroscopy for food quality analysis and control. Academic, San Diego, pp 16–23
Galvis-Sánchez AC, Santos IC, Mesquita RBR, Lopes JA, Rangel AOSS, Delgadillo I (2013) Food Anal Methods 6:470
Guillen MD, Cabo N (1997) J Am Oil Chem Soc 74:1281
Kays SE, Barton FE (2002) J Agric Food Chem 50:1284
Kays SE, Archibald DD, Sohn M (2005) J Sci Food Agric 85:1596
Kim Y, Kays SE (2009) J Agric Food Chem 57:8187
Kim Y, Kays SE, Himmelsbach DS (2007) J Agric Food Chem 55:4327
Kim SW, Min SR, Kim J, Park SK, Kim TI, Liu JR (2009) Plant Biotechnol Rep 3:87
Lin M, Rasco BA, Cavinato AG, Al-Holy M (2008) In: Sun DW (ed) Infrared spectroscopy for food quality analysis and control. Academic, San Diego, p 139
Maggio RM, Kaufman MT, Del Carlo M, Cerretani L, Bendini A, Cichelli A, Dario Compagnone D (2009) Food Chem 114:1549
Miller DS, Payne PR (1959) Brit J Nutr 13:501
Moros J, Inon FA, Salvador Garrigues S, de la Guardia M (2005) Anal Chim Acta 538:181
Ostrowska-Ligęza E, Górska A, Wirkowska M, Koczoń P (2012) J Therm Anal Calorim 110:137
Pettersson H, Aberg L (2003) Food Control 14:229
Rohman A, Sismindari, Erwanto Y, Che Man YB (2011) Meat Sci 88:91
Rzedzicki Z (2006) Bull IHAR 1:269
Silva SD, Rosa NF, Ferreira AE, Boas LV, Bronze MR (2009) Food Anal Methods 2:120
Smit LE, Schonfeldt HC, de Beer WHJ (2004) J Food Compos Anal 17:361
Syahariza ZA, Che Man YB, Selamat J, Bakar J (2005) Food Chem 92:365
van de Voort FR, Sedman J, Russin T (2001) Eur J Lipid Sci Technol 103:815
Vlachos N, Skopelitis Y, Psaroudaki M, Konstantinidou V, Chatzilazarou A, Tegou E (2006) Anal Chim Acta 573–574:459
Wang HL, Wan XY, Bi JC, Wang JK (2006) Cereal Chem 83:402
Conflict of Interest
Magdalena Reder declares that she has no conflict of interest. Piotr Koczoń declares that he has no conflict of interest. Magdalena Wirkowska declares that she has no conflict of interest. Katarzyna Sujka declares that she has no conflict of interest. Hanna Ciemniewska-Żytkiewicz declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Reder, M., Koczoń, P., Wirkowska, M. et al. The Application of FT-MIR Spectroscopy for the Evaluation of Energy Value, Fat Content, and Fatty Acid Composition in Selected Organic Oat Products. Food Anal. Methods 7, 547–554 (2014). https://doi.org/10.1007/s12161-013-9652-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9652-2