Abstract
The aim of this paper was to assess the oxidative stability of structured lipids synthesized by enzymatic interesterification of a blend of lard and rapeseed oil with concentrates of n − 3 fatty acids. Differential scanning calorimetry was used to evaluate the oxidation induction time of interesterified fats as a parameter assessing resistance of tested fats to their thermal-oxidative decomposition. Moreover, the IR spectra registered in the classic spectral range (4000–400 cm−1) were used to differentiate the samples of interesterified fats. The results show that the interesterification process decreased the induction time. Increased content polar fraction in the interesterified fatty product can reduce its resistance to oxidation. FT-IR data of selected spectral ranges correlate with the value of induction time at a statistically significant level. This is a proof that chemical changes occurring during different treatments of the starting mixture can be monitored by FT-IR spectroscopy. Moreover, obtained correlations can be used for the evaluation of an induction value of an unknown oil sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The composition and nature of oils and fats used for food preparation is more and more important due to both growing calorie consciousness of consumers and increasing incidences of cardiac problems. Nowadays, consumer demands concern fats and oils enriched in non-hydrogenated, low cholesterol, and polyunsaturated fatty acid [1, 2]. The current popularity of structured lipids arise from the fact that they are technologically and nutritionally improved lipids [3]. Structured lipids are triacylglycerols that have been modified to change the fatty acid composition and/or their positional distribution in the glycerol backbone by chemically and/or enzymatically catalyzed reactions and/or genetic engineering [4]. Enzymatic interesterification has received considerable attention in recent years. Traditionally, chemical catalysts have been used to conduct interesterification reactions. By replacing chemical catalysts with enzymes, acyl exchange can proceed in a controlled manner. Enzymatic interesterification can be used to upgrade cheap and saturated fats or to add value to commercial fats and oils [2, 5, 6].
Human milk is the major source of energy for many infants at the very beginning of their lives. It contains 2–6 % fat mainly as triacylglycerols which constitute about 50 % of the energy. Fatty acids present in human milk have a highly specific positional distribution of triacylglycerols, and this configuration has been implicated as the main factor determining the efficiency of human milk absorption. Pancreatic and gastric lipases selectively hydrolyze fatty acids at sn-1,3 positions, producing free fatty acids and 2-monoacylglycerols. 2-Palmitoylglycerol is more efficiently absorbed than palmitic acid in free form, the latter producing insoluble soaps with calcium and magnesium. The structure of lipids in human milk can be reproduced artificially by means of the enzymatic interesterification reaction using sn-1,3 specific lipase as a catalyst [4, 7–11].
Lard is an edible fat derived from pigs that is appreciated as an important ingredient for cooking [12]. This fat available in large quantities has the TAG structure similar to that found in human milk fats. Compared with human milk fat, lard contains less essential fatty acids, i.e., linoleic and linolenic acids. In most human milk fats, the ratio of linoleic acid to linolenic acid (LA/ALA) is generally between 5 and 15, while LA/ALA in lard is around 33 which differentiates it significantly from human milk fat. Taking this into consideration acyl donors should be rich in both linoleic and linolenic acids and have a correct ratio of LA/ALA to meet the requirements of infants [9, 13]. Human milk fat also contains very important essential fatty acids (EFA): docosahexaenoic (DHA) acid and arachidonic acid (AA) derived from the (n − 6) and (n − 3) EFA. These fatty acids are precursors for eicosanoids production which are powerful regulators of numerous cell and tissue functions. Human milk fat has an AA content of about 0.5 % and a DHA content of about 0.3 % of the total fatty acids [14]. It is very perspective and possible to incorporate polyunsaturated fatty acids (PUFA) from vegetable oils i.e., rapeseed oil and from fish oil into lard via lipase-catalyzed interesterification.
The successful production of structured lipids for infant milk formulation can be impeded by their high susceptibility to oxidative deterioration [11, 15, 16]. Structured lipids or infant milks produced with lipids containing unsaturated fatty acids can deteriorate during storage and produce off-flavors and odors characteristic of oxidation [3, 11, 17]. Furthermore, the production process for structured lipids increases free fatty acid (FFA) concentration, which is also responsible for off-flavor increase. A high concentration of FFA in infant milk induces a rancid and bitter taste that is unacceptable. Also, unsaturated fatty acids in infant milk fat can be oxidized rapidly into hydroperoxides, which can then be rapidly decomposed to the secondary oxidation products such as alkenes, aldehydes, and ketones [11, 17]. Oxidative deterioration and high FFA concentration can alter the nutritional quality of the structure of lipids [11, 18] and render infant milks containing these lipids potentially toxic and unacceptable to the consumers [11, 17].
The use of thermal analytical techniques and the information obtained is useful in controlling quality changes in food during processing and storage [19, 20]. IR spectroscopy serves as an established method to determine types and number of chemical bonds present in a sample [21] and, therefore, can be used to study chemical changes occurring in a processed fats.
The aim of this paper was to characterize and study the oxidative stability of structured lipids synthesized by enzymatic interesterification of a blend of lard and rapeseed oil with n − 3 concentrated fatty acids using differential scanning calorimetry (DSC) as well as using Fourier transform infrared spectroscopy (FT-IR).
Materials and methods
Materials
The mixtures of lard (L), rapeseed oil (RSO), and n − 3 fatty acids from fish in the form of their concentrates (ROPUFA), in proportions 7:2:1 were used in this investigation.
Lard and rapeseed oil were purchased commercially; ROPUFA 30 n − 3 FOOD Oil was purchased from DSM Nutritional Products.
Catalyst of enzymatic interesterification
Enzymatic interesterification was catalyzed by the commercial preparation Lipozyme RM IM, which contains immobilized lipase from Rhizomucor miehei. The fats were interesterified for 8 h, at a temperature of 60, 70, 80 °C. After a predetermined time, interesterification was stopped by filtering out the biocatalyst.
DSC measurements
A differential scanning calorimeter (DSC Q20 TA) coupled with a high-pressure cell (PDSC—pressure differential scanning calorimetry) was used. The instrument was calibrated using high-purity indium and tin metal standards. Samples of fat (3–4 mg) before and after interesterification were placed in an aluminum pan, under oxygen atmosphere, being pressurized in an isobaric module (1,400 kPa). The isothermal temperature (120 °C) was used for the data collection. Obtained diagrams were analyzed using TA Universal Analysis 2000 software. For each sample measurement was triplicate, the output was automatically recalculated and presented as amount of energy per 1 g.
FT-IR spectroscopy
IR spectra were registered in the classic range of 4,000–370 cm−1. 25 scans were collected for each sample of fat before and after interesterification with the 1 cm−1 resolution using a System 2000 Perkin Elmer spectrophotometer connected to PC software PeGrams running on Windows 98 platform. Samples of fat were registered as film between two KBr plates. Samples were placed on a plate as single drop of volume 0.05 mL. 10 spectra were registered for each sample, then an average spectrum was calculated and processed.
Determination of free fatty acids content
Acid values were determined by titration of fat samples dissolved in the mixture of ethanol:diethyl ether (1:1, v/v) with 0.1 M ethanolic potassium hydroxide solution.
FFA contents were calculated based on acid values and the values of average molar masses of fatty acids obtained from the results of gas chromatography (GC) determinations.
Column chromatography
Fats before and after interesterification were separated into triacylglycerols (TAG) and polar fraction (PF) by column chromatography on silica gel (SG 60, 70–230 mesh, Merck, Germany). The TAG were eluted with the mixture of petroleum ether:diethyl ether (87:13, v/v/) and then the PF fraction, that is FFA, monoacylglycerols and diacylglycerols, were eluted with diethyl ether. The percentages of the TAG and the PF were determined by weight after evaporation of eluting solvent.
GC measurements
The TAG obtained after column chromatography were analyzed for fatty acid composition. The determination of fatty acid composition was carried out by gas chromatographic (GC) analysis of fatty acid methyl esters. Methyl esters of fatty acids were prepared through saponification of TAG and esterification with methanol. An YL6100 GC chromatograph equipped with a flame ionization detector and MEGA-10 capillary column of 0.25 mm i.d. × 60 m length and 0.25 μm film thickness was used. The oven temperature was programmed as follows: 70 °C for 0.5 min, then it was increased by 15 °C min−1 to 160 °C; from 160 to 200 °C it was increased by 1.5 °C min−1; then kept at 200 °C for 15 min, from 200 to 225 °C it was increased by 30 °C, and then kept at 225 °C another 1 min. The temperature of the injector was 225 °C, with a split ratio of 1:100, and the detector temperature was 250 °C. Nitrogen flowing with the rate of 1.2 mL min−1 was used as the carrier gas. The identification of fatty acids was carried out using the lipid standard purchased from Sigma Aldrich.
Statistical analysis
Microsoft Excel 6.0 and Statgraphics Plus 4.0 were used for statistical evaluation of the experimental data.
Results and discussion
GC measurements
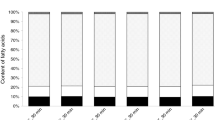
The results of the determination of fatty acid composition of lard, rapeseed oil, fish oil (ROPUFA), and the products of interesterification as well as human milk fat are presented in Fig. 1. Lard is mainly composed of saturated fatty acids (46.2 %), such as palmitic, and stearic acids and monounsaturated fatty acids (42.7 %) especially oleic acid. It also contains a significant quantity of linoleic acid (around 7 %). This is confirmed by De Leonardis et al. [12]. As a result of interesterification the unsaturated fatty acids from rapeseed oil and fish oil were incorporated into triacylglycerol structures of lard. TAG of interesterified fats contained from 14.0 to 14.9 % of PUFA. The content of PUFA in human milk is about 18 % of the total content of fatty acids [9]. The ratio of linoleic acid to linolenic acid in obtained structured lipids is between 4.1 and 4.9. The interesterified fats contain about 5 % of n − 3 EFA fatty acids, including docosahexaenoic acid and eicosapentaenoic acids. Therefore, interesterification reaction of blend of lard, rapeseed oil, and oil from fish allows for obtaining new fats that have a similar ratio of linoleic acid to linolenic acid to human milk fat, as well as containing a very important essential fatty acid from n − 3 group.
Content of fatty acids (SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids) for raw materials, for blends after interesterification, and for human milk fat. Values are derived from Xu [9]
Determination of free fatty acids and polar fraction content
The main component of fat are triacylglycerols. Fats contain also polar fraction (PF) which include monoacylglycerols, diacylglycerols, and FFAs. The composition of PF for mixtures before and after interesterification is presented in Fig. 2. The results show that after interesterification the content of PF increased. Blends esterified at the presence of Lipozyme RM IM contain 11.0–14.4 % of PF. The FFA content depends significantly on the temperature of the reaction (Fig. 3). The higher the temperature, the lower the FFA content. The natural function of lipases is to catalyze the hydrolysis of fats. They catalyze the hydrolysis of fats to give FFAs, partial acylglycerols, and glycerol. The reaction is reversible and the enzymes can be shown to catalyze the formation of acylglycerols from glycerol and FFAs under certain conditions. If the water level is reduced, however, some lipases will continue to catalyze reactions and at a certain level interesterification begins to dominate over hydrolysis [22, 23].
DSC measurements
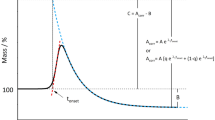
Increased content PF in the interesterified fatty product can reduce its resistance to oxidation [23]. Oxidative stability of oils and fats is one of the most important parameters for their quality assessment. A number of methods for such an assessment have been developed [24], among which DSC is one of the most frequently used [25, 26]. The results of PDSC measurements, expressed as the oxidation induction time are shown in Fig. 4. The induction time obtained for each of the analyzed fats can be used as the primary parameter for the assessment of the resistance of tested fat to its oxidative decomposition. Generally, samples with higher induction time are more stable than those for which the induction time obtained at the same temperature is lower [23]. The PDSC tests performed for interesterified fats showed that induction time was reduced compared to the starting blend. In general, most studies have reported a decrease in oxidative stability of interesterified fats compared to the initial mixture [3, 11]. According to most studies, the different methods of production or purification of structured lipids, the different nature of oil sources, or the presence of antioxidants during the manufacturing are within the main factors that determine the oxidative stability of structured lipids. In addition, the molecular structure of the TAG, such as fatty acid composition, positional distribution on the glycerol backbone, as well as the interaction of both factors, are also important parameters that influence the stability of the structured products [3]. The induction time of interesterified fats depends on the temperature of the reaction. Optimum temperature of 8-h interesterification of this kind of mixture, with use of Lipozyme RM IM as catalyst, is 80 °C. The content of PF of products of interesterification conducted at this temperature is the lowest.
Spectral analysis
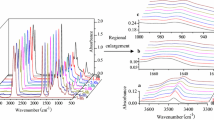
Obtained average spectra for each of following samples are fairly similar one to another as general; however, detailed differences distinguishing spectra were observed in some spectral ranges. Those differences are obviously due to different chemical composition of reacting mixture. Each of the compounds present in the mixture as well as its amount influences the course of spectrum. Therefore, even small quality and quantity differences can be monitored by FT-IR spectrum course. In the 2nd column of Table 1 seven selected ranges are presented. According to the literature data, in the spectral range in which band intensity is sensitive to the changes of oxidative stability of the mixture in the highest degree, bands are most probably generated by =C–H deformations (see Table 1 raw 3). Change of oxidation state and, therefore, oxidative stability is mainly related to number and location of double bonds, which in turn influence the =C–H deformations. Total area and maximum height in given spectral range are presented in column 3 an 4, respectively. Column 5 contains data of induction time, obtained by PDSC method. The attempt was made to correlate spectral and calorimetric data. Column 6 and 7 present correlation coefficients calculated between two independent datasets. In the case of spectral range 640–626 cm−1, highest correlation coefficients for both area and maximum height were obtained. Detailed route of spectra of four average samples in this range are presented in Fig. 5, while linear correlation between spectral area and induction time is present on Fig. 6.
Conclusions
Structured lipids or infant milks produced with lipids containing unsaturated fatty acids can deteriorate during storage and produce off-flavors and odors characteristic of oxidation. The oxidation induction time obtained from DSC measurements can be used as parameters for the assessment of the resistance of tested fats to its thermal-oxidative decomposition. Results of the researches show that interesterification has influenced on reduction of the induction time. It is clearly seen that spectral data of selected ranges correlate with the value of induction time in a very high degree, while other regions are not sensitive or related to induction time. This is a proof that chemical changes undergoing during different treatment of starting mixture can be monitored by FT-IR spectroscopy. Moreover, obtained correlations can be used for evaluation of induction value of unknown oil sample.
Abbreviations
- LRSO:ROPUFA:
-
Mixture of lard, rapeseed oil, and n − 3 fatty acids (concentrates) in proportions 7:2:1
- 60 °C/8 h:
-
Mixture interesterified for 8 h at 60 °C
- 70 °C/8 h:
-
Mixture interesterified for 8 h at 70 °C
- 80 °C/8 h:
-
Mixture interesterified for 8 h at 80 °C
References
Gupta R, Rathi P, Bradoo S. Lipase mediated upgradation of dietary fats and oils. Crit Rev Food Sci Nutr. 2003;43(6):635–44.
Jeyarani T, Yella Redy S. Effect of enzymatic interesterification on physicochemical properties of mahua oil and kokum fat blend. Food Chem. 2010;123(2):249–53.
Martin D, Reglero G, Senorans FJ. Oxidative stability of structured lipids. Eur Food Res Technol. 2010;231:635–53.
Claro da Silva R, Schaffer De Martini Soares FA, Hazzan M, Capacla IR, Almeida Goncalves MI, Gioielli LA. Continuous enzymatic interesterification of lard and soybean oil blend: Effects of different flow rates on physical properties and acyl migration. J Mol Catal B Enzym. 2012;76:23–8.
Forssell P, Kervinen R, Lappi M, Linko P, Suoptti T, Poutanen K. Effect of enzymatic interesterification on the melting point of tallow–rapeseed oil (LEAR) mixture. JAOCS. 1992;69:126–9.
Seriburi V, Akoh CC. Enzymatic interesterification of triolein and tristearin: chemical structure and differential scanning calorimetric analysis of the products. JAOCS. 1998;75(6):1339–45.
Lien EL. The role of fatty acid composition and positional distribution in fat absorption in infants. J Pediatr. 1994;125:562–8.
Forsyth JS. Lipids and infant formulas: a review. Nutr Res. 1998;11:255–78.
Xu X. Production of specific-structured triacylglycerols by lipase-catalyzed reactions: a review. Eur J Lipid Sci Technol. 2000;102:287–303.
Sahin N, Akoh CC, Karaali A. Enzymatic production of human milk fat substitutes containing γ-linolenic acid: optimization of reactions by response surface methodology. JAOCS. 2005;82:549–57.
Maduko C, Park Y, Akoh CC. Characterization and oxidative stability of structured lipids: infant milk fat analog. JAOCS. 2008;85:197–204.
De Leonardis A, Macciola V, Giuseppe Lembo G, Aretini A, Nag A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007;100:998–1004.
Yang T, Xu X, He C, Li L. Lipase-catalyzed modification of lard to produce human milk fat substitutes. Food Chem. 2003;80:473–81.
Koletzko B, Thiel I, Abiodun PO. The fatty acid composition of human milk in Europe and Africa. J Pediatr. 1992;120:S62–70.
Jennings BH, Akoh CC. Enzymatic modification of triacylglycerols of high eicosapentaenoic and docosahexaenoic acids content to produce structured lipids. JAOCS. 1999;76:1133–7.
Akoh CC, Moussata CO. Characterization and oxidative stability of enzymatically produced fish and canola oil-based structured lipids. JAOCS. 2001;78:25–30.
Nielsen NS, Yang T, Xu X, Jacobsen Ch. Production and oxidative stability of a human milk fat substitute produced from lard by enzyme technology in a pilot packed-bed reactor. Food Chem. 2006;94:53–60.
Osborn HT, Akoh CC. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid-based oil-in-water emulsions. Food Chem. 2003;84:451–6.
Roos Y. Thermal analysis, state transitions and food quality. J Therm Anal Calorim. 2003;71:197–203.
Vivoda M, Roškar R, Kmetec V. The development of a quick method for amorphicity determination by isothermal microcalorimetry. J Therm Anal Calorim. 2011;105:1023–30.
Ostrowska-Ligęza E, Górska A, Wirkowska M, Koczoń P. An assessment of various powdered baby formulas by conventional methods (DSC) or FT-IR spectroscopy. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-011-2158-5.
Liu L, Lampert D. Monitoring chemical interesterification. JAOCS. 1999;76:783–7.
Kowalski B, Tarnowska K, Gruczynska E, Bekas W. Chemical and enzymatic interesterification of beef tallow and rapeseed oil blend with low content of tallow. J Oleo Sci. 2004;53(10):479–88.
Kowalski B, Gruczynska E, Maciaszek K. Kinetics of rapeseed oil oxidation by pressure differential scanning calorimetry measurements. Eur J Lipid Sci Technol. 2000;102:337–41.
Tan CP, Che Man YB. Recent developments in differential scanning calorimetry for assessing oxidative deterioration of vegetable oils. Trends Food Sci Technol. 2002;13:312–8.
Wirkowska M, Ostrowska-Ligęza E, Górska A, Koczoń P. Thermal properties of fats extracted from powdered baby formulas. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-012-2245-2.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education Grant No. N N312 068439. The results of this research were presented at the XXXIV AICAT Congress 2012.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bryś, J., Wirkowska, M., Górska, A. et al. The use of DSC and FT-IR spectroscopy for evaluation of oxidative stability of interesterified fats. J Therm Anal Calorim 112, 481–487 (2013). https://doi.org/10.1007/s10973-012-2794-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2794-4