Abstract
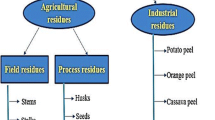
Enzymatic catalysts, such as lipases, have been extensively studied due to their promise as an alternative to chemical catalysts. They offer advantages like biodegradability (green biotechnology) and the potential for enzyme recycling (turnover), leading to reduced operational costs. The primary objective of this study was to produce lipase from the fungus Penicillium polonicum through solid-state fermentation, utilizing agro-industrial waste as substrate. The aim was to apply the obtained lipase as a biological catalyst in the synthesis of ethyl oleate ester. In the lipase production process, the filamentous fungus P. polonicum, along with sunflower seed cake (SSC) and rice husk (RH), served as substrate and support, respectively, for solid-state fermentation (SSF). Experiments involved varying proportions of both substrate and support (0%, 25%, 50%, 75%, and 100% (RH) and 100% (SSC)). Subsequently, the impact of glycerol as an inducer (1%, 3%, and 5%, with a 25/75% SSC/RH ratio) at SSF was investigated. The obtained results revealed a catalytic activity of 29.3 U g−1 under optimal conditions: 55% moisture, 25/75% SSC/RH, and at 27 °C during 96 h of fermentation. The lipase produced was employed as an enzymatic catalyst in studies involving the synthesis of ethyl oleate ester in n-heptane, utilizing experimental design 23. Variables such as temperature, enzymatic activity, and molar ratio (alcohol/acid) were modified. The best experimental conditions for the enzymatic synthesis of ethyl oleate ester were determined to be an alcohol/acid molar ratio of 6:1, a temperature of 37 °C, and an enzymatic activity of 60 U. This resulted in 100% conversion into ester within 5 h of reaction time. The outcomes demonstrated that lipase effectively catalyzed the synthesis of ethyl oleate, a biodiesel ester, with a high yield.






Similar content being viewed by others
References
Höfer R (2015) Sugar-and starch-based biorefineries. In: Industrial biorefineries and white biotechnology, pp 157–235
Punekar NS (2018) Enzymes: catalysis, kinetics, and mechanisms. Springer. https://doi.org/10.1007/978-981-13-0785-0
Thangaraj B, Solomon PR, Muniyandi B, Ranganathan S, Lin L (2019) Catalysis in biodiesel production—a review. Clean Energy 3(1):2–23. https://doi.org/10.1093/ce/zky020
Thapa S, Li H, Ohair J, Bhatti S, Chen FC, Nasr KA, Johnson T, Zhou S (2019) Biochemical characteristics of microbial enzymes and their significance from industrial perspectives. Mol Biotechnol 61(8):579–601. https://doi.org/10.1007/s12033-019-00187-1
Fatima S, Faryad A, Ataa A, Joyia FA, Parvaiz A (2020) Microbial lipase production: a deep insight into the recent advances of lipase production and purification techniques. Biotechnol Appl Biochem 68(3):445–458. https://doi.org/10.1002/bab.2019
Priyanka P, Tan Y, Kinsella GK, Henehan GT, Ryan BJ (2019) Solvent stable microbial lipases: current understanding and biotechnological applications. Biotechnol Lett 41(2):203–220. https://doi.org/10.1007/s10529-018-02633-7
Lee LP, Karbul HM, Citartan M, Gopinath SC, Lakshmipriya T, Tang TH (2015) Lipase-secreting Bacillus species in an oil-contaminated habitat: promising strains to alleviate oil pollution. BioMed res inter. https://doi.org/10.1155/2015/820575
Vyas S, Chhabra M (2017) Isolation, identification and characterization of Cystobasidium oligophagum JRC1: a cellulase and lipase producing oleaginous yeast. Bioresour Technol 223:250–258. https://doi.org/10.1016/j.biortech.2016.10.039
Gutarra ML, Godoy MG, Maugeri F, Rodrigues MI, Freire DM, Castilho LR (2009) Production of an acidic and thermostable lipase of the mesophilic fungus Penicillium simplicissimum by solid-state fermentation. Bior tec 100:5249–5254. https://doi.org/10.1016/j.biortech.2008.08.050
Mahmoud GA, Koutb MM, Morsy FM, Bagy MM (2015) Characterization of lipase enzyme produced by hydrocarbons using fungus Aspergillus terreus. Eur J Biol Res 5:70–77
Çakmak M, Aydoğdu H (2021) Screening of microfungi for lipolytic activity and optimization of process parameters in lipase production by solid substrate fermentation using selected microfungi (Penicillium aurantiogriseum). Kuwait J Sci 48:98–105
Sukma A, Jos B, Sumardiono S (2018) Kinetic of biomass growth and protein formation on rice bran fermentation using Rhizopus oryzae. In: MATEC Web of Conferences. EDP Sci 156:01023. https://doi.org/10.1051/matecconf/201815601023
Vandenberghe LP, Pandey A, Carvalho JC, Letti LA, Woiciechowski AL, Karp SG, Thomaz-Soccol S, Martínez-Burgos WJ, Penha RO, Hermann LW, Soccol CR (2021) Solid-state fermentation technology and innovation for the production of agricultural and animal feed bioproducts. Syst Microbiol Biomanuf 1(2):142–165
Bharathi D, Rajalakshmi G (2019) Microbial lipases: an overview of screening, production and purification. Biocatal Agric Biotechnol 22:101368. https://doi.org/10.1016/j.bcab.2019.101368
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5(1):1–15. https://doi.org/10.1186/s40643-017-0187-z
Kachrimanidou V, Kopsahelis N, Chatzifragkou A, Papanikolaou S, Yanniotis S, Kookos I, Koutinas AA (2013) Utilization of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valorization 4:529–537. https://doi.org/10.1007/s12649-012-9191-x
Adıgüzel AO (2020) Production and characterization of thermo-, halo-and solvent-stable esterase from Bacillus mojavensis TH309. Biocatal Biotransformation 38(3):210–226. https://doi.org/10.1080/10242422.2020.1715370
Putri DN, Khootama A, Perdani MS, Utami TS, Hermansyah H (2020) Optimization of Aspergillus niger lipase production by solid state fermentation of agro-industrial waste. Energy Rep 6:331–335. https://doi.org/10.1016/j.egyr.2019.08.064
Kumar A, Kanwar SS (2012) Lipase production in solid-state fermentation (SSF): recent developments and biotechnological applications. Dyn Biochem Process Biotechnol and Mol Bio 6(1):13–27
Paluzar H, Tuncay D, Aydogdu H (2021) Production and characterization of lipase from Penicillium aurantiogriseum under solid-state fermentation using sunflower pulp. Biocatal Biotransformation 39(4):333–342. https://doi.org/10.1080/10242422.2021.1901888
Maldonado RR, Lopes DB, Aguiar-Oliveira E, Kamimura ES, Macedo GA (2016) A review on Geotrichum lipases: production, purification, immobilization and applications. CABEQ 4:439–454
Quayson E, Amoah J, Hama S, Kondo A, Ogino C (2020) Immobilized lipases for biodiesel production: current and future greening opportunities. Renew Sustain Energy Rev 134:110355. https://doi.org/10.1016/j.rser.2020.110355
Dickel JDM, Carvalho JK, Silveira MAD, Menegotto dos Santos P, Rodrigues MLF, Fagundes-Klen MR, Rosa CA, Buzanello CV, Lucca RAS, Santos ARO, Da Rosa MF (2022) Aspergillus sclerotiorum lipolytic activity and its application in bioremediation of high-fat dairy wastewater environments. Environ Sci Pollut Res Int 1–11. https://doi.org/10.1007/s11356-022-24669-z.
AOAC - Association of Official Analytical Chemists (2005) Official methods of analysis of AOAC International, 18th edn. AOAC, Washington
Lowry RR, Tinsley JI (1976) Rapid colorimetric determination of free acids. J Am Oil Chem Soc 53:470–472. https://doi.org/10.1007/BF02636814
Shu ZY, Jiang H, Lin RF, Jiang YM, Lin L, Huang JZ (2010) Technical methods to improve yield, activity and stability in the development of microbial lipases. J Mol Catal B Enzym 62:1–8. https://doi.org/10.1016/j.molcatb.2009.09.003
Fernandes MLM, Saad EB, Meira JA, Ramos LP, Mitchell DA, Krieger N (2007) Esterification and transesterification reactions catalysed by addition of fermented solids to organic reaction media. J Mol Catal B Enzym 44:8–13. https://doi.org/10.1016/j.molcatb.2006.08.004
Rodrigues MLF, Da Silva EA, Borba CE, Oliveira ACD, Kruger C, Raimundo RW, Silva LP, Vanzin M, Stuani BT (2015) Produção de enzimas hidrolíticas pelo fungo endofítico Penicillium sp. Isolado das folhas de Ricinus communis L. Rev Bras Energias Renováveis 4:129–145
Haq IU, Mukhtar H, Umber H (2006) Production of protease by Penicillium chrysogenum through optimization of environmental conditions. J Agri Soc Sci 2(1):23–25 (1813–2235/2006/02–1–23–25)
Soccol CR, Costa ESF, Letti LAJ, Karp SG, Woiciechowski AL, Vandenberghe LPS (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Innov 1(1):52–71. https://doi.org/10.1016/j.biori.2017.01.002
Rattanapoltee P, Dujjanutat P, Muanruksa P, Kaewkannetra P (2021) Biocircular platform for third generation biodiesel production: batch/fed batch mixotrophic cultivations of microalgae using glycerol waste as a carbon source. Biochem Eng J 75:108128. https://doi.org/10.1016/j.bej.2021.108128
Dasari MA, Kiatsimkul PP, Sutterlin WR, Suppes GJ (2005) Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal A 228(1):225–231. https://doi.org/10.1016/j.apcata.2004.11.033
Alcala-Galiano DDM, López JAC, Borbón ER, González JAR, Cuadros RC, Ramos-Sánchez LB (2021) Condiciones para la transesterificación enzimática de aceite de Jatropha curcas con un sólido fermentado. Centro Azúcar 48(2):56–67
Oliveira BH, Coradia GV, Oliva-Neto P, Nascimento VMG (2020) Biocatalytic benefits of immobilized Fusarium sp. (GFC) lipase from solid state fermentation on free lipase from submerged fermentation. Ind Crops Prod 147:112235. https://doi.org/10.1016/j.indcrop.2020.112235
Oliveira ACD, Frenscha G, Marquesa FA, Vargasa JVC, Rodrigues MLF, Mariano AB (2020) Production of methyl oleate by direct addition of fermented solid Penicillium sumatrense and Aspergillus fumigatus. Renew Energy 162:1132–1139
Zhong L, Feng Y, Wang G, Wang Z, Bilal M, Lv H, Hexin LV, Jia CJ (2020) Production and use of immobilized lipases in/on nanomaterials: a review from the waste to biodiesel production. Int J Biol Macromol 152:207–222. https://doi.org/10.1016/j.ijbiomac.2020.02.258
Carvalho JK, Krüger C, Silveira MAD, Piana PA, Rodrigues MLF, Rosado AF, Lucca RAS, Fagundes-Klein MR, da Silva, EA, Buzanello CV, Teleken JG, Zanella RA (2024) Lipolytic production from solid-state fermentation of the filamentous fungus Penicillium polonicum and its applicability as biocatalyst in the synthesis of ethyl oleate. Envir Sci and Pol Res, 1–12. https://doi.org/10.1007/s11356-024-33007-4.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jéssyca Ketterine Carvalho, Ricardo Antonio Zanella, Maria Luiza Fernandes Rodrigues, Adriana Fiorini Rosado, and Pitágoras Augusto Piana. The first draft of the manuscript was written by Jéssyca Ketterine Carvalho, Ricardo Antonio Zanella, Pitágoras Augusto Piana, Adriana Fiorini Rosado, Mairim Dahm da Silva, Rosemeire Aparecida da Silva de Lucca, Marcia Regina Fagundes-Klen, Edson Antônio da Silva, Karine Zanella, Cleide Viviane Buzanello, Álvaro Barcellos Onofrio, and Maria Luiza Fernandes Rodrigues, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carvalho, J.K., Zanella, R.A., Piana, P.A. et al. Production of Fermented Solid Containing Lipases from Penicillium polonicum and Its Direct Use as Biocatalyst in the Synthesis of Ethyl Oleate. Bioenerg. Res. (2024). https://doi.org/10.1007/s12155-024-10772-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12155-024-10772-1