Abstract
Pinus pinea nuts are commercial relevant Mediterranean edible forest nuts, with an increasing production and market value, whose industrial processing yields a lignocellulosic by-product, the pine nut shells, currently only used for combustion. Little research has been done on pine nut shells that could support a value-added application for this residue. This work studies for the first time the production of oligosaccharides by autohydrosis, and aims at an integrated upgrade within the biorefinery framework. Autohydrolysis was explored in the temperature range between 150 and 230 °C (corresponding to severity factors 2.13–4.63). Oligosaccharides, mainly xylo-oligosaccharides (95% of the total), were the key soluble products, reaching 28.7 g/100 g of xylan of the feedstock at the optimal conditions (log R0 4.01). Other products were monosaccharides and phenolic compounds that reached 7.8 and 4.7 g/L, respectively, under the most severe conditions. The stability of the oligosaccharides at different temperatures (room, 37 °C and 100 °C) and pH (between 1 and 11) grant them significant market potential in the food and pharma sectors. The pre-treated pine nut shells by autohydrolysis presented an improved, although low, enzymatic digestibility (14%), and an improved high-heating value, therefore advising their further valorization by thermochemical pathways.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pinus pinea L. (stone pine) is a softwood species, cultivated in a total area estimated at 600,000 ha, distributed in the Mediterranean region and particularly important in the Iberian Peninsula due to its adaptability to moist but well-drained soils in coastal sandy areas suffering little temperature variation [1]. Although P. pinea trees may be used for timber production, they are mostly valued for production of their edible seed kernels, the pine nuts, which have a significant economic importance and a high, and increasing, market value. Production of pine nuts represents the most valuable and profitable activity for the stone pine plantations in Spain, Portugal, Italy, and Turkey, increasing the economic development of the rural areas where they grow.
The commercial production of pine nuts starts with the field collection of the pinecones where the seeds are enclosed. In the processing mills, the pinecones are opened and the seeds extracted, followed by the crushing of the hard lignocellulosic shells that enclose the pine kernels or pinions, which are the high-valued commercial product. The pine nut shells represent about 77% of the seed mass and make up an agro-industrial by-product that is produced in large quantities and concentrated at the nut-processing mill, thereby enabling their easy management [2]. The use of side mass flows from production of edible nuts is increasingly receiving attention under the concept of full resource use and zero-waste chains, and many research works have studied, e.g., almond, walnut or peanut shells, among others. A recent report estimated a world availability of waste pine nut shells of 114 thousand tons per year [3].
Comparatively to other nut shells, less studies have considered pine nut shells and evaluated their potential for production of added value products, and currently, they are used only as a low added-value energy source directly for combustion. Most existing works are related to the thermochemical conversion for the production of bio-oil [4], activated carbons [5], and biochar [6] while steam distillation was tested to produce essential oils with application in foodborne diseases [7]. There are only a few works related to the chemical characterization of this hard and brittle material, reported to have a high lignin content of 40.5%, a significant proportion of xylan in the hemicellulosic fraction (xylose amounts to 65% of the non-glucose monosaccharides), a moderate content of extractives (4.5%), and low ash [2], thereby chemically differing from other nut shells. A very recent study with P. pinea pine nut shells from Turkey also reported low ash (1.7%) and extractives (4.2%) and a high Klason lignin content (52.2%) [3]. As such, the chemical composition of the pine nut shells suggests a potential valorization based on the upgrade of its hemicelluloses and lignin fractions.
The biochemical platform of biorefineries is an advantageous pathway to produce high value-added products, namely focused on the valorization of hemicelluloses that requires a selective fractionation of the structural macromolecular components. Hydrothermal processes such as autohydrolysis have been one of the best choices for this purpose since no chemicals other than water are used, and limited equipment corrosion and low by-product generation are expected to occur. Autohydrolysis has been applied on several biomass materials, allowing a high recovery of solubilized hemicelluloses, specifically as oligosaccharides [8, 9]. Monomeric sugars, acetic acid, and phenolic compounds are also obtained together with a minor production of sugar degradation compounds (e.g., furfural, 5-hydroxymethylfurfural, formic, and levulinic acids) [10]. The solid lignocellulosic fraction remaining after autohydrolysis, containing cellulose and lignin, can undergo further valorization, namely through delignification processes and cellulose enzymatic hydrolysis to produce glucose, and lignin-derived phenolic compounds, while also not excluding a direct use for energy, e.g., in co-generation [11, 12].
The composition and structure of the oligosaccharides obtained by autohydrolysis, namely regarding chain length, branching degree, monosaccharide composition, and overall purity in the liquid stream, are influenced by raw material and process conditions, thereby allowing targeted production for a wide range of applications [13]. Many studies have focused on xylo-oligosaccharides (XOS) since they have biological activities such as antiallergy, anti-microbial, anti-infection, and anti-inflammatory properties, selective cytotoxic activity, immunomodulatory action, cosmetic, and a variety of other properties [14]. The food and feed industries are also targeting novel carbohydrates as new prebiotic oligosaccharides that may act as nutrients for the probiotic Bifidobacteria and Lactobacilli [15, 16] and can be recognized as functional oligosaccharides [17]. Furthermore, they also are used as low-sweetness humectants [14, 17, 18]. Studies have also shown that XOS have the potential to reduce serum cholesterol and triglycerides levels which are important indicators of cardiovascular disorders [19].
Pine nut shells have not yet been researched as a potential feedstock along such a biorefinery approach. The aim of this work is to evaluate the selective production of hemicellulosic oligosaccharides from pine nut shells using hydrothermal treatments (autohydrolysis) under various process conditions using the severity factor (log R0) as treatment intensity measure. The results were analyzed in terms of yields obtained in the autohydrolysis liquors for oligosaccharides, monosaccharides, and by-products, and for cellulose and lignin recovery in the autohydrolysis remaining solids. Studies of oligosaccharides stability in the liquor and digestibility of the remaining solids was also conducted. The results presented in this work will be the first to be published for pine nut shells from Pinus pinea and aim at contributing to their valorization as a resource within a chemical platform of biorefineries.
Material and Methods
Feedstock
Pine nut shells from Pinus pinea L. were supplied by one industrial company in Portugal, as obtained from the nut processing. The pine nut shells were coarsely ground with a knife mill (Waring, Snijders Scientific, Holland), and screened for particle size characterization. The fractions retained between 1.00- and 3.55-mm wire openings were collected, homogenized in a single lot, and stored in a capped PE-plastic container, at room temperature.
Autohydrolysis
The pine nut shells were mixed with water at a liquid-to-solid ratio of 3 (w/w, dry basis), to a total mass of 1.2 kg. A 2-L stainless steel reactor (Parr Instruments Co., Illinois, USA) with PID temperature controller (4842, Parr Instruments Co., Illinois, USA) was used for the autohydrolysis. The reaction was carried out under continuous agitation (150 rpm) and in non-isothermal operation up to 230 °C with a typical heating rate of 3.8 °C/min. Reaction was stopped when the desired final temperature was attained for the values between 150 to 230 °C.
After reaction completion, a rapid cooling was made (typically less than two minutes to reach temperatures below 100 °C), and the liquid and solid streams were separated by filtration (Whatman Nr. 41 filter paper), under vacuum. The solid residues were washed with 500 mL of distilled water and filtered once again. The effect of the treatment conditions is interpreted based on the severity factor, log R0, taking into account the full temperature profile, as detailed elsewhere [20] with \(R0={\int }_{0}^{t}\mathrm{exp}\left(\frac{T(t)-100}{14.75}\right)dt\) where the temperature T (°C) is a function of time t (min), and 14.75 is an empirical parameter related with activation energy and temperature. The following log R0 conditions were tested: 2.13, 2.80, 3.42, 4.01, and 4.63, corresponding to a maximum temperature of 150, 170, 190, 210, and 230 °C respectively.
Chemical Analytical Procedures
The chemical composition of the pine nut shells feedstock and of the solids remaining after autohydrolysis were determined using standard procedures provided by the National Renewable Energy Laboratory (NREL) [21].
The composition of the autohydrolysis’ liquid stream was carried out by HPLC with an Aminex HPX-87H column to quantify free monosaccharides, formic, acetic and levulinic acids, and furan derivatives (furfural and 5-hydroxymethylfurfural). Since galactose and mannose are co-eluted at the same time of xylose, therefore not allowing individual peak separation, it was assumed that the peak is mostly xylose [2].
Monosaccharides and aliphatic acids were quantified using a refractive index detector (RID), while furans were quantified using a diode array detector (DAD) using data obtained at 280 nm. Oligosaccharides were quantified indirectly after their hydrolysis to monomers using an aliquot of liquor subjected to quantitative acid hydrolysis. The methodology followed previously detailed protocols [21, 22].
The total phenolic compounds content of the hydrolysates was assayed spectrophotometrically at 765 nm by the Folin–Ciocalteu method [23] using a microplate spectrophotometer (Multiskan™ GO, Thermo Scientific, MA, USA) and gallic acid as calibration standard. The phenolic compounds were identified by capillary zone electrophoresis (CZE), as previously described [24].
Stability of Oligosaccharides in the Liquid Fraction
Shelf life of the liquid fraction and of the solubilized oligosaccharides was measured in the hydrolysate obtained in optimal conditions. A defined volume was filtered through 0.45-μm Millipore® filters and kept in a dry place at room temperature away from light. At specific intervals (once a week), a sample was taken for chemical analysis to quantify the oligosaccharides content and optical microscopy inspection for the presence of microbial contaminants.
The oligosaccharides’ stability to pH and temperature was also evaluated by applying a method previously developed in our laboratory [25], based on methods described in the literature [26]. First, the liquors were filtered (Millipore® filters with pore diameter 0.45 μm), divided into 500-mL wide-mouthed flasks and freeze-dried using a Labconco (USA) freeze-dryer, at – 35 °C. These purified OS were used for all the tests.
The oligosaccharides samples were dissolved in “buffer” solutions (Table 1), resulting in a concentration of approximately 100 g/L, and pH 1, and 3. The prepared solutions were placed in a thermostatic oil bath at 100 °C, during 1 h, and subsequently analyzed by HPLC to determine the stability of the oligosaccharides.
Enzymatic Digestibility
The enzymatic digestibility of the feedstock and processed solids was carried out based on the NREL/TP-510–42,629 protocol [27]. This procedure consists in using a sample of biomass of 0.15 g that is digested in the presence of 5 mL of sodium citrate 0.1 M pH 4.8 buffer solution, 100 μL of a solution of sodium azide (2% w/v), as anti-microbial agent and Celluclast® 1.5 L, and Novozyme 188 enzymes in prescribed quantities to attain 60 FPU/g and 64 pNPGU/g of dry biomass, respectively. The volume was adjusted with distilled water to 10 mL considering that the biomass has a density of 1. Two blank assays were also prepared, to consider both the (i) sugars that may arise from the enzyme solution (blank assay carried out without added biomass), and (ii) the sugars derived from non-enzymatical biomass hydrolysis (blank assay carried out without adding enzymes) to correct the results for the free saccharides present in the biomass and products that could possibly be formed in the absence of enzymes.
The samples were placed in a 50-mL plastic, round-bottom, capped centrifuge tubes, and incubated at 50 °C, under orbital shaking (150 rpm) in an incubator (Comecta, Spain). After 72 h, the tubes were placed in a boiling water bath, for 5 min, to inactivate the enzymes. Finally, the samples were filtrated using nylon membranes (0.22 μm) and analyzed by HPLC for the released sugars. All assays were performed, at least, in duplicate. The enzymatic digestibility was quantified by the ratio of digested cellulose to the initial cellulose content.
Results and Discussion
The pine nut shells had the following chemical composition as determined using standard chemical summative analysis: 4.75% proteins, 3.04% ash, 2.67% extractives, 42.63% Klason lignin, 25.93% cellulose (as glucan), 20.98% hemicelluloses (as the sum of 16.27% xylan, galactan and mannan, 2.72% arabinan, and 1.99% acetyl groups).
The chemical composition of pine nut shells was previously determined by Queirós et al. (2019) and the reported values are similar to those obtained here, e.g., 4.5% extractives, 40.5% lignin, and 48.7% polysaccharides with the following composition: glucose 29.1%, xylose 12.7%, mannose 4.0%, galactose 1.2%, arabinose 1.2%, rhamnose 0.1%, 1.2% galacturonic acid 0.2%, and acetyl groups 0.3% [2]. Sen et al. (2022) also reported a similar chemical composition with 4.2% extractives and 52.2% Klason lignin [3].
Effect of Autohydrolysis on the Composition of the Liquid Phase
Pine nut shells were subjected to the autohydrolysis process under non-isothermal conditions to final temperatures of 150, 170, 190, 210, and 230 °C, corresponding to severity factors of log R0 2.13, 2.80, 3.42, 4.01, and 4.63 respectively. Figure 1 shows the composition of the autohydrolysis liquor at the tested conditions as a function of the reaction severity (log R0), regarding concentration of xylo-oligosaccharides and gluco-oligosaccarides (Fig. 1A), of the monosaccharides glucose, xylose, and arabinose, and acetic acid (Fig. 1B), and of furfural and 5-hydroxymethylfurfural (Fig. 1C).
Concentration profile (g/L) of oligosaccharides, monosaccharides, acetic acid, and degradation products as a function of log R0. plot A:
 hemicellulosic oligosaccharides,
hemicellulosic oligosaccharides,
 gluco-oligosaccharides; plot B: Monomers
gluco-oligosaccharides; plot B: Monomers
 xylose,
xylose,
 glucose,
glucose,
 arabinose,
arabinose,
 acetic acid; plot C:
acetic acid; plot C:
 5-hydroxymethylfurfural,
5-hydroxymethylfurfural,
 furfural,
furfural,
 formic acid, and
formic acid, and
 levulinic acid. Lines are used for eye guidance only
levulinic acid. Lines are used for eye guidance only
Oligosaccharides were the main compounds in the liquor, especially for the intermediary severities, reaching a maximum of 16.5 g/L at log R0 4.01, which corresponds to 6.6% of the initial pine nut shell mass. The majority of the hemicellulosic oligosaccharides solubilized by autohydrolysis were xylo-oligosaccharides containing mainly xylose, and smaller proportions of arabinose, galactose, mannose, and of acetyl groups, reflecting the initial composition of the pine nut shells hemicelluloses. Gluco-oligosaccharides accounted for less than 5% of the total. Xylo-oligosaccharides concentration increased markedly from 0.98 g/L at log R0 2.0 to 14.88 g/L at log R0 4.01 (210 °C), corresponding to the increased xylan depolymerization into soluble oligomers with higher temperatures, but decreased abruptly after that to 1.10 g/L at R0 4.5, corresponding to the hydrolysis of the solubilized oligomers to monosaccharides. At the optimal conditions of log R0 4.01, the solubilization of xylo-oligosaccharides corresponds to a 28.74 g/100 g of xylan in the pine nut shell feedstock. This value compares with results obtained with eucalyptus wood but it is relatively low in relation to other biomasses like corn straw with values above 40% [8].
Nevertheless, the present results demonstrated that autohydrolysis of pine nut shells is a possible process to produce xylo-oligosaccharides, as it uses a low liquid-to-solid ratio of 3 with low loss of efficiency. This is a significant advantage in terms of cost reduction, as compared to the common use of liquid-to-solid ratios of 7 to 10, as described in the literature [8, 10]. Low liquid-to-solid ratios (i.e., high solid loadings) offer many advantages since larger biomass amounts can be processed per unit operation, thereby allowing less capital costs in biorefinery implementation. Furthermore, the process will consume lower amounts of water and energy, making it more environmental and economic sustainable and also simplifying downstream processing [28].
The decrease of xylo-oligosaccharides concentration for the most severe condition tested is due to their depolymerization into monosaccharides and subsequent degradation products, as shown in Fig. 1. This has been observed in other autohydrolysis studies for severities above 4.0 and is consistent with previous results on wood, agricultural, and agro-industrial residues [8, 28,29,30,31].
The carbohydrate monomers resulting from depolymerization of hemicelluloses or of xylo-oligosaccharides increased gradually with severity, reaching a maximum of 6.40 g/L at log RO 4.67, mainly consisting of xylose (Fig. 1B). Similarly, xylose concentration increased from log R0 3.42 onwards, and acetic acid started to accumulate reaching 3.53 g/L, that corresponds to 94.8% of all the acetyl groups present in xylan.
Furfural and 5-hydroxymethylfurfural are degradation products of pentose and hexose sugars, respectively. Under the highest oligosaccharides-yielding conditions, both these furans had low concentrations, higher for furfural as compared to 5-hydroxymethylfurfural due to the higher amounts of pentose sugars in pine nut shell hemicelluloses, and the higher susceptibility of pentoses to undergo dehydration [26]. Other sugar degradation by-products such as levulinic and formic acid were also present, but in low concentrations, so that the total acid concentration (including acetic acid) for the optimal xylo-oligosaccharides production condition was below 2 g/L. Furans and acids are potential microbial inhibitors but their low concentration in the autohydrolysis liquors does not preclude their use for fermentation processes [32].
The hydrolysates were also characterized by the Folin–Ciocalteu method for quantification of the total phenolic compounds (Fig. 2), which mainly derive from lignin degradation [33].
The profile shows a significant increase in the content of phenolic compounds with increasing severities, reaching a maximum concentration of 4.7 g/L for the most severe condition tested at log R0 4.67. These results are consistent with previous studies on wood and other agricultural and agro-industrial residues, e.g., eucalyptus residues, wheat straw, and olive tree prunings that also yielded phenolic concentrations above 4 g/L for similar severities [34] and corn straw with a maximum phenolic concentration of 6.97 g/L at log R0 4.51 [8]. The total content of phenolic compounds under the optimal conditions for xylo-oligosaccharides production does not preclude the use of the hydrolysis liquors in fermentation processes.
Several phenolic compounds with potential added-value applications, namely catechol, vanillin, vanillic acid, and 3-hydroxybenzoic acid, were identified in the hydrolysis liquors using CZE, especially for higher severity factors (log R0 ≥ 3.42). Although these compounds may be produced with higher yields by organosolv or alkaline delignification processes [11], the concentrations obtained in this work are significant, given the low liquid-to-solid ratio used. These compounds can be easily separated from the oligosaccharides by simple purification processes, such as membrane filtration [24].
Hydrolysate’s Shelf Life Evaluation
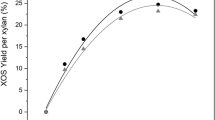
A possible industrial application of oligosaccharides must rely on their stability which is not trivial when they are stored in a liquid phase for a long time [35]. The storage stability of the liquor obtained under the optimal autohydrolysis condition at log R0 4.0 was studied at room temperature in a dark and dry place, without any type of microbial sterilization. Figure 3 shows the concentration of oligosaccharides present in the liquor depending on the storage time.
All oligosaccharides were stable during storage for at least 3 weeks, and no microbial contamination was detected. A similar evaluation of oligosaccharides stability was reported for Annona cherimoya hydrolysates with comparable results [25]. The absence of contamination in the liquors can be a consequence of the presence of furans, acids, and phenolic compounds, that can present a synergistic inhibitory effect of microbial growth [33, 36].
Oligosaccharides’ Stability
Oligosaccharides are mainly used in the food and feed sectors as prebiotic supplements, typically up to 10% (by weight) [35]. Their food use requires that they are as free as possible from metabolic inhibitors, such as furans (that are known carcinogenic and hepatoxic compounds) and aliphatic acids. Freeze-drying was tested as a liquor concentration and drying process for the oligosaccharides. This process also allowed removal of 89% and 85% of 5-hydroxymethylfurfural and furfural, respectively, and of 66% of acetic acid.
The stability of oligosaccharides was studied as a function of pH (1, 2, 7, and 11), and temperature (37 °C and 100 °C) by measuring the concentration in glucose and xylose before and after the stability tests. An increase of their concentration would mean a depolymerization of the oligosaccharides. The stability profiles of xylo-oligosaccharides and gluco-oligosaccharides at 100 °C for 1 h as a function of pH are shown on Fig. 4.
Both xylo-oligosaccharides and gluco-oligosaccharides were quite stable in the tested pH range. This is a particularly interesting result since oligosaccharides obtained from wheat bran or chicory were not so stable at pH 3 and 11, mainly those derived from chicory, which were sensitive to alkaline decomposition [35]. The oligosaccharides obtained from pine nut shells are therefore suitable for applications in the food industries and likely to be processed industrially.
The oligosaccharides ability to pass the human gut and reach the intestine should be evaluated when considering food applications, knowing that the stomachal digestive process occurs at 37 °C, for about 3 h, and pH values between 1 and 3. Under these conditions, the produced xylo-oligosaccharides showed no significant hydrolysis (data not shown). This was predictable according to the stability reported for other comparable compounds at 25, 37, and 50 °C under pH 1, 2, and 3 [37, 38], and this is also a first guarantee that their potential nutritional properties may be maintained when passing the digestive process.
Furthermore, these data also support that the purification of the obtained oligosaccharides may not require the freeze-drying step and potentially be achieved by evaporation or spray drying, which have significant techno-economic advantages.
Effect of Autohydrolysis on the Composition of the Solids
Within a biorefinery framework, after the autohydrolysis treatment, it is necessary to find an application for the remaining biomass. Figure 5 shows the composition of the initial pine nut shells feedstock and of the processed solids after autohydrolysis with the different severity factors.
The mass loss, i.e., the solid solubilization induced by the autohydrolysis process was very low for the less severe conditions and the solids remaining showed a polysaccharide and lignin content very similar to that of the initial feedstock (Fig. 5). However, with the severity factor log R0 3.42, the solid yield decreased sharply to 79.8%. This decrease results from solubilization of hemicelluloses which increased for the higher temperature tested, although the hemicelluloses were not completely removed due to the presence of slow-reacting xylan [39]. Conversely, arabinan was totally removed from the solid phase as well as acetyl groups that appeared in high concentration as free acetic acid in the hydrolysate for log R0 4.63 (Fig. 1).
The relative amounts of lignin and glucan in the solid residues increased with the autohydrolysis severity, i.e., the autohydrolyzed solids were enriched in lignin and cellulose. This is consistent with previous results showing a low effect of autohydrolysis on the solubilization of cellulose and lignin that therefore remain in the solid [8, 40].
A potential valorization route for the autohydrolyzed solids is their use for glucose production by cellulose hydrolysis, namely by enzymatic hydrolysis. Therefore, the enzymatic digestibility of the remaining cellulose in the autohydrolyzed solids is important for this evaluation. For this, enzymatic tests were performed using the enzymes Celluclast 1.5 L and Novozyme 188, and the results obtained for the autohydrolyzed solids with the different severity factors are shown in Fig. 6. The enzymatic digestibility increased slightly with autohydrolysis severity but reached only 14% of saccharification yield for the most severe treatment (log R0 4.63), and low glucose concentrations were obtained (1.07 g/L). The digestibility is lower than that reported for other materials with similar pre-treatment and enzymatic hydrolysis conditions, e.g., with the same enzymes, banana pseudostem pulp showed 90% digestibility [41], Annona cherimoya seeds 83% digestibility [25], and olive tree prunings and eucalyptus residues respectively 64.8% and 49.8% digestibility [34]. This high recalcitrance of pine nut shell cellulose to enzymatic hydrolysis may be partly explained by the high lignin content of the processed pine nut shells, as it is significantly higher as compared to the other reported materials as well as by the fact that it is a G-lignin [2]. Similar results were very recently published showing that extensive ball milling was required for lignin isolation by cellulose enzymatic hydrolysis from pine nut shells due to their recalcitrance [42]. The substrate capacity to adsorb the enzymes is also important and the compact structure of pine nut shells may not allow a suitable enzyme accessibility [43]. These results suggest that a delignification step after autohydrolysis and prior to the enzymatic digestion might be beneficial as it has been described for other materials [44].
Alternatively, the remaining cellulose and lignin-rich solid can be advantageously upgraded in the thermochemical platform of the biorefinery, targeting energy products [3,4,5,6]. Finally, combustion remains a possible utilization pathway, with the advantage given by an increased biomass high-heating value (HHV), favored by the high lignin and low hemicellulose content, and estimated at 19.0 kJ/kg [45].
Conclusions
Pine nut shells can be processed by autohydrolysis at a low liquid-to-solid ratio to produce oligosaccharides, mainly xylo-oligosaccharides, in the liquid fraction under mild conditions (log R0 4.0) while higher severity autohydrolysis conditions (log R0 > 4.6) extensively solubilized the hemicelluloses into monomers and promoted sugar degradation. The produced oligosaccharides kept in the hydrolysate were stable at room temperature for a 3-week period, without previous sterilization, and were chemically stable at 100 °C for 1 h and at 37 °C for 3 h, assuring the firsts traits for a potential use in the prebiotic market.
The autohydrolyzed pine nut shells were cellulose-lignin matrices showing recalcitrance towards cellulose digestibility, therefore directing their further valorization towards thermochemical pathways.
Data Availability
The data sets generated during the current study are available from the corresponding author upon reasonable request.
References
Jaouadi W, Alsubeie M, Mechergui K, Naghmouchi S (2020) Silviculture of Pinus pinea L. in North Africa and the mediterranean areas: current potentiality and economic value. J Sust For:1–19. https://doi.org/10.1080/10549811.2020.1798787
Queirós CSGP, Cardoso S, Lourenço A, Ferreira J, Miranda I, Lourenço MJV, Pereira H (2019) Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Bio Conv Bioref 10:175–188. https://doi.org/10.1007/s13399-019-00424-2
Sen AU, Correia R, Longo A, Nobre C, Alves O, Santos M, Gonçalves M, Miranda I, Pereira H (2022) Chemical composition, morphology, antioxidante, and fuel properties of pine nut shells within a biorefinery perspective. Biom Conv Bioref. https://doi.org/10.1007/s13399-022-03605-8
Kim Y-M, Kim S, Han TU, Park Y-K, Watanabe C (2014) Pyrolysis reaction characteristics of Korean pine (Pinus koraiensis) nut shell. J Anal Appl Pyrolysis 110:435–441. https://doi.org/10.1016/j.jaap.2014.10.013
Uçar S, Karagöz S (2014) Co-pyrolysis of pine nut shells with scrap tires. Fuel 137:85–93. https://doi.org/10.1016/j.fuel.2014.07.082
Chen D, Chen X, Sun J, Zheng Z, Fu K (2016) Pyrolysis polygeneration of pine nut shell: quality of pyrolysis products and study on the preparation of activated carbon from biochar. Biores Technol 216:629–636. https://doi.org/10.1016/j.biortech.2016.05.107
Liu R, Xu B (2012) Characterization of essential oil in pine nut shells from commodity waste in China by steam distillation and GC-MS. Food Anal Meth 5:435–440. https://doi.org/10.1007/s12161-011-9264-7
Moniz P, Pereira H, Quilhó T, Carvalheiro F (2013) Characterisation and hydrothermal processing of corn straw towards the selective fractionation of hemicelluloses. Ind Crop Prod 50:145–153. https://doi.org/10.1016/j.indcrop.2013.06.037
Carvalheiro F, Esteves MP, Parajó JC, Pereira H, Gírio FM (2004) Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Biores Technol 91(1):93–100. https://doi.org/10.1016/S0960-8524(03)00148-2
Alves-Ferreira J, Duarte LC, Lourenço A, Roseiro LB, Fernandes MC, Pereira H, Carvalheiro F (2019) Distillery residues from Cistus ladanifer (rockrose) as feedstock for the production of added-value phenolic compounds and hemicellulosic oligosaccharides. BioEner Res 12(2):347–358. https://doi.org/10.1007/s12155-019-09975-8
Alves-Ferreira J, Lourenço A, Morgado F, Duarte LC, Roseiro LB, Fernandes MC, Pereira H, Carvalheiro F (2021) Delignification of Cistus ladanifer biomass by organosolv and alkali processes. Energies 14(4):1127. https://doi.org/10.3390/en14041127
Silva-Fernandes T, Duarte LC, Carvalheiro F, Loureiro-Dias MC, Fonseca C, Girio F (2015) Hydrothermal pretreatment of several lignocellulosic mixtures containing wheat straw and two hardwood residues available in Southern Europe. Biores Technol 183:213–220. https://doi.org/10.1016/j.biortech.2015.01.059
Moure A, Gullón P, Domínguez H, Parajó JC (2006) Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Proc Biochem 41(9):1913–1923. https://doi.org/10.1016/j.procbio.2006.05.011
Patel S, Goyal A (2011) Functional oligosaccharides: production, properties and applications. W J Microb Biotechnol 27(5):1119–1128. https://doi.org/10.1007/s11274-010-0558-5
Wang Y, Lu J, Zhou S, Du J, Tao Y, Cheng Y, Wang H (2022) Bioconversion of cellulose and hemicellulose in reed sawdust to xylo-oligosaccharides and L-lactic acid. Ind Crop Prod 187:115390. https://doi.org/10.1016/j.indcrop.2022.115390
Ríos-Ríos KL, Rémond C, Dejonghe W, Van Roy S, Vangeel S, Van Hecke W (2022) Production of tailored xylo-oligosaccharides from beechwood xylan by different enzyme membrane reactors and evaluation of their prebiotic activity. Biochem Eng J 185:108494. https://doi.org/10.1016/j.bej.2022.108494
Mussatto SI, Mancilha IM (2007) Non-digestible oligosaccharides: a review. Carbohyd Polym 68(3):587–597. https://doi.org/10.1016/j.carbpol.2006.12.011
de Freitas C, Terrone CC, Forsan CF, Milagres AMF, Brienzo M (2022) Oligosaccharides from lignocellulosic biomass and their biological and physicochemical properties. In: Brienzo M (ed) Hemicellulose Biorefinery: a sustainable solution for value addition to bio-based products and bioenergy. Springer Nature, Singapore, pp 275–309. https://doi.org/10.1007/978-981-16-3682-0_9
Sharma R, Kataria A, Sharma S, Singh B (2022) Structural characterisation, biological activities and pharmacological potential of glycosaminoglycans and oligosaccharides: a review. Int J Food Sci Technol 57(1):4–15. https://doi.org/10.1111/ijfs.15379
Overend RP, Chornet E (1987) Fractionation of lignocellulosics by steam-aqueous pretreatments. Philosoph Trans Royal Soc London Series A-Math Phys Eng Sci A321:523–536. https://doi.org/10.1098/rsta.1987.0029
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass: laboratory analytical procedure. NREL/TP-510–42618. National Renewable Energy Laboratory, Golden, CO
Sluiter A, Hames B, Ruiz R, Scarlata CJ, Sluiter J, Templeton D (2008) Determination of sugars, byproducts, and degradation products in liquid fraction process samples: laboratory analytical procedure. NREL/TP: 510-42623, National Renewable Energy Laboratory, Golden, CO
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxid Antioxid Pt A 299:152–178. https://doi.org/10.1016/S0076-6879(99
Moniz P, Serralheiro C, Matos CT, Boeriu CG, Frissen AE, Duarte LC, Roseiro LB, Pereira H, Carvalheiro F (2018) Membrane separation and characterisation of lignin and its derived products obtained by a mild ethanol organosolv treatment of rice straw. Process Biochem 65:136–145. https://doi.org/10.1016/j.procbio.2017.11.012
Branco PC, Dionísio AM, Torrado I, Carvalheiro F, Castilho PC, Duarte LC (2015) Autohydrolysis of Annona cherimoya mill. seeds: optimization, modeling and products characterization. Biochem Eng J 104:2–9. https://doi.org/10.1016/j.bej.2015.06.006
Danon B, Marcotullio G, de Jong W (2014) Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem 16(1):39–54. https://doi.org/10.1039/C3GC41351A
Selig M, Weiss N, Ji Y (2008) Enzymatic saccharification of lignocellulosic biomass: laboratory analytical procedure. NREL/TP:510-42629. National Renewable Energy Laboratory, Golden, CO
Carvalheiro F, Duarte LC, Gírio F, Moniz P (2016) Hydrothermal/liquid hot water pretreatment (autohydrolysis) a multipurpose process for biomass upgrading. In: Mussatto SI (ed) Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier, Amsterdam, pp 315–347. https://doi.org/10.1016/B978-0-12-802323-5.00014-1
Ho AL, Carvalheiro F, Duarte LC, Roseiro LB, Charalampopoulos D, Rastall RA (2014) Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Biores Technol 152:526–529. https://doi.org/10.1016/j.biortech.2013.10.114
Parajó JC, Garrote G, Cruz JM, Dominguez H (2004) Production of xylooligosaccharides by autohydrolysis of lignocellulosic materials. Trends Food Sci Technol 15(3–4):115–120. https://doi.org/10.1016/j.tifs.2003.09.009
Garrote G, Dominguez H, Parajo JC (2004) Production of substituted oligosaccharides by hydrolytic processing of barley husks. Ind Eng Chem Res 43(7):1608–1614. https://doi.org/10.1021/ie0342762
Duarte LC, Carvalheiro F, Neves I, Girio FM (2005) Effects of aliphatic acids, furfural, and phenolic compounds on Debaryomyces hansenii CCMI 941. Appl Biochem Biotechnol 121–124:413–425. https://doi.org/10.1385/abab:121:1-3:0413
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Biores Technol 74(1):25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
Silva-Fernandes T, Duarte LC, Carvalheiro F, Marques S, Loureiro-Dias MC, Fonseca C, Girio F (2015) Biorefining strategy for maximal monosaccharide recovery from three different feedstocks: eucalyptus residues, wheat straw and olive tree pruning. Biores Technol 183:203–212. https://doi.org/10.1016/j.biortech.2015.01.136
Courtin CM, Swennen K, Verjans P, Delcour JA (2009) Heat and pH stability of prebiotic arabinoxylooligosaccharides, xylooligosaccharides and fructooligosaccharides. Food Chem 112(4):831–837. https://doi.org/10.1016/j.foodchem.2008.06.039
Duarte LC, Carvalheiro F, Tadeu J, Gírio FM (2006) The combined effects of acetic acid, formic acid, and hydroquinone on Debaryomyces hansenii physiology. Appl Biochem Biotechnol 129–132(1–3):461–475. https://doi.org/10.1385/abab:130:1:461
Rumpagaporn P, Kaur A, Campanella OH, Patterson JA, Hamaker BR (2012) Heat and pH stability of alkali-extractable corn arabinoxylan and its xylanase-hydrolyzate and their viscosity behavior. J Food Sci 77:H23–H30. https://doi.org/10.1111/j.1750-3841.2011.02482.x
Wang Q, Ellis PR, Ross-Murphy SB (2000) The stability of guar gum in an aqueous system under acidic conditions. Food Hydrocoll 14(2):129–134. https://doi.org/10.1016/S0268-005X(99)00058-2
Garrote G, Domínguez H, Parajó JC (2002) Autohydrolysis of corncob: study of non-isothermal operation for xylooligosaccharide production. J Food Eng 52 (3):211–218. Pii S0260–8774(01)00108-X
Nabarlatz D, Ebringerová A, Montané D (2007) Autohydrolysis of agricultural by-products for the production of xylo-oligosaccharides. Carbohyd Polym 69:20–28. https://doi.org/10.1016/j.carbpol.2006.08.020
Díaz S, Ortega Z, Benítez AN, Costa D, Carvalheiro F, Fernandes MC, Duarte LC (2021) Assessment of the effect of autohydrolysis treatment in banana’s pseudostem pulp. Waste Manag 119:306–314. https://doi.org/10.1016/j.wasman.2020.09.034
Wang Z, Zhu X, Deuss PJ (2021) The effect of ball milling on birch, pine, reed, walnut shell enzymatic hydrolysis recalcitrance and the structure of the isolated residual enzyme lignin. Ind Crop Prod 167:113493. https://doi.org/10.1016/j.indcrop.2021.113493
Ge S, Wu Y, Peng W, Xia C, Mei C, Cai L, Shi SQ, Sonne C, Lam SS, Tsang YF (2020) High pressure CO2 hydrothermal pretreatment of peanut shells for enzymatic hydrolysis conversion into glucose. Chem Eng J 385:123949. https://doi.org/10.1016/j.cej.2019.123949
Moniz P, João L, Duarte LC, Roseiro LB, Boeriu CG, Pereira H, Carvalheiro F (2015) Fractionation of hemicelluloses and lignin from rice straw by combining autohydrolysis and optimised mild organosolv delignification. BioResources 10:2626–2641. https://doi.org/10.15376/biores.10.2.2626-2641
Álvarez A, Pizarro C, García R, Bueno JL (2015) Spanish biofuels heating value estimation based on structural analysis. Ind Crop Prod 77:983–991. https://doi.org/10.1016/j.indcrop.2015.09.078
Acknowledgements
The authors gratefully acknowledge the experimental support of Céu Penedo and Belina Ribeiro.
Funding
Open access funding provided by FCT|FCCN (b-on). Ivone Torrado received the PhD grant from Fundação para a Ciência e a Tecnologia (FCT), Portugal, in the SUSFOR doctoral program (PD/BD/114175/2016). FCT financed the projects supporting Centro de Estudos Florestais, MED, and CHANGE (UID/AGR/00239/2020, UIDB/05183/2020, and LA/P/0121/2020 respectively). Part of this work was carried out at the Biomass and Bioenergy Research Infrastructure (BBRI), funded by the BBRI-LISBOA-01–0145-FEDER-022059 project supported by the Operational Programme for Competitiveness and Internationalization (PORTUGAL2020), and the regional operational programs Lisboa 2020 and Norte 2020, through the European Regional Development Fund. The work was also supported by the QREN Project “Biomassa Endógena”.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and laboratorial analysis were performed by Ivone Torrado, Ana Dionísio, and Luísa Bivar Roseiro. The first draft of the paper was written by Ivone Torrado and subsequently improved by Helena Pereira, Luís Duarte, and Maria C. Fernandes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torrado, I., Dionísio, A., Fernandes, M.C. et al. Production of Oligosaccharides from Pine Nut Shells by Autohydrolysis. Bioenerg. Res. 16, 2253–2261 (2023). https://doi.org/10.1007/s12155-023-10585-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10585-8