Abstract
Pine nut shells, a biomass residue from the Mediterranean Pinus pinea pine nut industrial processing, were treated by microwave-assisted autohydrolysis to produce xylo-oligosaccharides. Microwave-assisted processes provide alternative heating that may reduce energy input and increase overall process efficiency. The autohydrolysis treatments were performed under isothermal and non-isothermal operations within a wide range of operational conditions (temperature/reaction times) covering several severity regimes (as measured by the log R0 severity factor). The composition of the autohydrolysis liquors was determined in terms of oligo- and monosaccharides, aliphatic acids and degradation compounds. The process was highly selective towards hemicelluloses hydrolysis and liquid streams containing a mixture of oligomeric compounds (mainly xylo-oligosaccharides) could be obtained under relatively mild operation conditions (190 °C, 30 min) with a maximal oligosaccharides’ concentration of 18.48 g/L. The average polymerization degree of the obtained oligosaccharides was characterised by HPLC, showing that for the optimal conditions a mixture of oligomers with DPs from 2 to 6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pinus pinea L., stone pine or umbrella pine, is a typical Mediterranean forest species, mostly relevant in Portugal and Spain (especially in their southern regions) where about 70% of the P. pinea pine nut world production concentrates. The pine nut seeds have a thick ligneous shell that encloses the pine kernel and represents about 77% of the weight of the pine nuts [1]. These shells are potential biomass sources that have the logistical advantage of being concentrated at the industrial processing facility [2]. However, they are currently only used as a low added-value energy source for direct combustion without any valorisation upgrade technologies.
Pine nut shells have a high content of lignin and polysaccharides, with low extractives and ash content, which suggest possible processing routes in the biorefinery framework, namely targeted to a polysaccharide-based conversion [1]. The hemicelluloses consist mainly of xylans with very low acetylation and uronic units and a low proportion of galactoglucomannans. Hemicelluloses may be converted into oligosaccharides, compounds that have been increasingly valued in food and pharmaceutical industries, and have received a large attention in recent research [3,4,5].
Conversion of lignocellulosic biomass to biofuels and value-added bio-products has been attracting much attention in the research and industrial sectors, although some constraints exist for the economic feasibility in biorefineries [3, 4]. The development of efficient, cost-effective and eco-friendly pretreatment processes is one challenge in biomass conversion [6]. Pretreatments can be physical (mechanical, radiation), chemical (e.g. alkali, acid, organosolv, ionic liquid-based) or physical-chemical (e.g. steam explosion, ammonia fibre explosion). Hydrothermal processes such as autohydrolysis present several technological advantages and are therefore one of the best biomass pretreatment options [7]. No chemicals other than water are used, and low equipment corrosion and by-product generation are expected to occur, but it requires high temperature, typically in the range of 150–250 °C. The present energy context advises the use of alternative heating techniques that may reduce the energy input and increase the total process efficiency.
In this framework, microwave processing has attracted attention both from the R&D and industrial sectors since it satisfies many requirements of green chemistry [8]. Microwaves are electromagnetic irradiation in the frequency range of 0.3 to 300 GHz, whose energy per photon is less than 10−3 eV, even at the highest frequency. This is too low to induce ionization and to break chemical bonds and has also lesser energy than the Brownian motion [9]. Microwaves apply an electromagnetic field directly to the molecular structure of the heated material leading to physical, chemical or biological reactions. Microwave-assisted extraction is one of the techniques that allows faster and larger extractions of several compounds, including phenolic compounds, with several advantages over other methods [10, 11]. Among other advantages, microwaves allow the rapid heating of aqueous mixtures with non-ionising electromagnetic radiation, lower solvent use, greater selectivity for targeted compounds, better efficiency and lower extraction times in comparison with other heating systems (e.g. 10 times less), thereby decreasing the energy consumption and allowing additional automation [12].
The efficient heating of materials by microwave dielectric heating effects is the basis for microwave-enhanced chemistry [13]. This is very attractive for many chemical applications and is now widely accepted as a non-conventional energy source for performing organic synthesis as shown by the increasing number of related publications in recent years and with the general availability of new and reliable microwave instrumentation [14]. Microwave heating has been successfully applied in biomass pretreatments where in addition to the heating process it causes swelling and fragmentation [15,16,17].
This work studies the use of microwave-assisted hydrothermal pretreatment of pine nut shells under various isothermal and non-isothermal conditions for the production of oligosaccharides and analyses their composition and polymerization degree.
2 Experimental
2.1 Feedstock
Pine nut shells were provided by a Portuguese pine nut processing industry. Upon reception, the shells were milled with a benchtop grinder and screened to obtain a homogenous size lot (1–3.55 mm). The material was stored in vacuum-sealed bags until required for processing or analysis. Granulometric characterization of the milled sample was carried out in a sieve shaker (Endecotts, England) with sieves from 1.00 to 4.00 mm. The material (approximately 100 g) was screened for 20 min, and the fraction retained on each sieve was weighed to quantify the respective mass fraction. These assays were carried out in duplicate.
2.2 Microwave-assisted autohydrolysis
The microwave-assisted autohydrolysis assays were performed in a Microwave Digestor (Ethos Easy, Milestone Srl, Italy) using a magnetron frequency of 2450 MHz and EasyWave software for monitoring and control. Pine nut shells were reacted with water in capped Teflon vessels, with a loading of 15 g of dry biomass and 45 g of water, corresponding to a liquid-to-solid ratio of 3:1 (mass ratio of H2O/biomass). Several isothermal and non-isothermal conditions were tested, and the microwave power was continuously varied to maintain the prescribed temperature profile. The overall temperature profiles were recorded for all treatments (see below).
In non-isothermal conditions, the reactors were heated to a final temperature between 170 and 230 °C at a rate of 3.6 °C/min, as in a conventional non-isothermal autohydrolysis process [18]. In isothermal assays, the temperature was fixed at 190 °C which was attained also with a rate of 3.6 °C/min, and different isothermal periods between 0 and 60 min were tested. After reaching the targeted temperature or reaction period time, the reactors were rapidly cooled by immersion in an ice bath. All assays were carried out in triplicate.
The separation of the liquid and solid streams was made by using filtering crucibles under vacuum. The liquid fraction was further filtered through quantitative filter paper (Whatman nr. 41) to remove any remaining solids. The solid fraction was washed with two volumes (90 g each) of water, filtered and frozen before chemical composition analysis. For comparison purposes, the severity of the treatments was estimated as summatory of heating, isothermal and cooling step calculating the log R0, based on the measured temperature profile data for each step [19] according to Eq. (1).
2.3 Chemical composition analysis
The initial pine nut shells and the solid residues obtained after microwave-assisted autohydrolysis were characterised following standard NREL protocols [20]. Shortly, this characterization consists firstly in dissolving 0.3g of material to analyse in 3 mL 72% (w/w) sulfuric acid at 30 °C for 1h. Afterward, the acid concentration is diluted to 4% (w/w) and then all mixture is subjected to a heating process in an autoclave for 1h at 121 °C.
The liquid fraction was analysed in relation to solubilised monosaccharides (glucose, xylose and arabinose), aliphatic acids (formic, acetic and levulinic) and furan derivatives (5-hydroxymethylfurfural and furfural) by high-pressure liquid chromatography using a Thermo Scientific system (USA), equipped with a refractive index detector (Refractomax521) controlled at 39 °C and a diode array detector (DAD 3000), using a Bio-Rad Aminex HPX-87H column (300 × 7.8 mm) (Hercules, CA). The mobile phase was H2SO4 5 mM, the column temperature 50 °C, the flow rate 0.6 mL min−1 and the injection volume was 5 μL. Mannose was quantified in the same chromatographic system using a column REZEX RPM-Monosaccharide (Pb2+, 8% cross-linked Pb2+; 300 × 7.8 mm, 9 μm; Phenomenex) at a column temperature of 85 °C and a flow rate of 0.6 mL/ min. All samples were filtered with Millipore® (Cork, Ireland) 0.45 μm cellulose acetate membrane filters prior to analysis.
The oligosaccharides were determined indirectly after quantitative acid hydrolysis according to NREL/TP-510-42623 [21]. Concentrated sulfuric acid (72%) was added to an aliquot of the liquor resulting from the autohydrolysis treatments, in order to attain a 4% H2SO4 concentration, and hydrolysed in an autoclave at 121 °C for 1 h. After completion, the hydrolysates were slowly cooled down to room temperature, a sample was collected, filtered using 0.45 μm membranes (Millipore) and analysed by HPLC. The oligosaccharide concentration was calculated from the increase in sugar monomers in relation to their direct determination in the liquor. This procedure was performed in duplicate.
2.4 Polymerization degree of oligosaccharides
For characterization of the polymerization degree of oligosaccharides, liquors were injected in a REZEX RSO-Oligosaccharide (Phenomenex, Torrance, CA, USA) column filled with a sulphonated polystyrene-divinylbenzene resin in the Ag+-form (200 × 10.00 mm I.D., 4% cross-linking, particle size 8 μm), equipped with a guard column with the same filling, with the column at 75 °C. Ultrapure water at 0.3 mL/min was used as an eluent. Calibration for polymerization degree (PD) was carried out using a light corn syrup sample (mainly composed of malto-oligosaccharides) from Phenomenex. The calibration curve obtained is presented as supplementary material.
3 Results and discussion
3.1 Pine nut shell characterization
A granulometric characterization was performed after the size reduction of the pine nut shells (Fig. 1). Very few fines were produced in accordance with the brittleness of the pine shells. The 2.36 to 3.55 mm fraction accounted for more than 45% of the material and particles ranging from 1.00 to 3.55 mm corresponded to 75% of the total mass. This particle diameter range is within what is described in the literature as the most suitable for autohydrolysis processes [22].
The chemical characterization of the pine nut shells is detailed in Table 1, together with values reported in other studies for pine nut shells of P. pinea [1, 23] and Pinus koraiensis [24].
The pine nut shells used in this study have a high lignin content (44.5%), and a significant content of polysaccharides, including cellulose (25.9%, estimated as glucan), and hemicelluloses (18.2%, as the sum of xylan, mannan, arabinan and acetyl groups). This composition is similar to values found in the literature for pine nut shells of P. pinea [25] and Pinus koraiensis [24, 25].
The pine nut shell hemicelluloses comprise mostly xylans with low proportions of arabinose units and acetyl substitutions, corresponding to about 13.1% of the material. This is not typical for softwood species for which galactoglucomannans are the main wood hemicelluloses. Mannan is however still significant, being almost 30% of the hemicellulosic sugars. Galactose was not detected and non-cellulosic glucose was not determined. Although Queirós et al. [1] obtained 1.2% of galactose, the methodology used in the present work (NREL vs TAPPI) did not allow its detection. Also, it must be kept in mind that there could a variability in the composition of the pine nut shell. On the other hand, the pine nut shell may not have the same hemicellulosic composition as softwood species despite galactoglucomannans being its major component [26].
The chemical characterization of biomass materials is determined for evaluation of their potential for the biochemical/chemical platform of biorefineries. A significant content in hemicelluloses allows considering it for the production of oligosaccharides, as it occurs in the present study.
3.2 Microwave-assisted autohydrolysis under non-isothermal conditions
The microwave-assisted autohydrolysis process was studied using non-isothermal and isothermal conditions. The heating profiles for the various experiments are linear and overlap, showing a high degree of reproducibility of the equipment operating conditions, as shown in the Supplementary material (S1).
The pine nut shells were subjected to microwave-assisted autohydrolysis treatments under non-isothermal conditions, i.e. the reaction was stopped when the desired final temperature was attained. Final temperatures between 170 and 230 °C were tested, corresponding to the following severities: log R0 2.82 (170 °C), log R0 3.45 (190 °C), log R0 3.78 (200 °C), log R0 4.10 (210 °C), log R0 4.66 (230 °C), log R0 5.17 (250 °C). The heating profile was similar to that used in a previous study using standard autohydrolysis of pine nut shells [18] carried out in a Parr reactor with electric heating through an external mantle. Figure 2 shows the composition of the autohydrolysis liquor as a function of the severity factor.
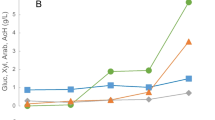
Composition (g/L) of the liquid fractions obtained after non-isothermal microwave-assisted autohydrolysis of pine nut shells as a function of log R0: A filled diamond, xylo- and mannan-oligosaccharides; filled triangle, arabino-oligosaccharides; filled square, gluco-oligosaccharides; B filled square, glucose; filled diamond, xylose and mannose; filled triangle, arabinose; C filled circle, acetic acid; filled triangle, formic acid; empty circle, levulinic acid; filled diamond, furfural; filled square, HMF. Lines are used for eye guide only
Oligosaccharides (OS) were the most relevant products, reaching a total concentration of 6.4 g/L for log R0 4.66, which corresponds to a yield of 1.59% (g XOS/100g pine nut shells). The hemicellulose-derived oligosaccharides contain xylose, arabinose and mannose as main sugars, and acetyl groups, reflecting the composition of the pine nut shells hemicelluloses. They are here referred to as xylo-oligosaccharides as xylose accounts for the majority of the hemicellulosic sugars. Gluco-oligosaccharides are present with a low concentration corresponding to 5% of the total oligosaccharides.
The concentration of xylo-oligosaccharides increased from 0.16 to 5.96 g/L and decreased after that. The severity condition leading to the highest production of oligosaccharides (log R0 4.66) is higher than for other lignocellulosic materials that typically present values close to 4 [27, 28]. Pine nut shells are more recalcitrant to hydrolysis and will require more severe operational conditions at the industrial level.
For the most severe condition tested (log R0 5.17), the xylo-oligosaccharide concentration decreased abruptly to 0.89 g/L, as a result of their depolymerization to monosaccharides and the degradation of monosaccharides to aliphatic acids and furans, which increased significantly. Such carbohydrate behaviour in autohydrolysis has been reported for several biomass materials and is typically observed for severities above 4.01 [29,30,31]. This is also consistent with studies on wood and other agricultural and agro-industrial residues reporting the autohydrolysis selectivity towards hemicellulose depolymerisation [32, 33].
The results show that autohydrolysis has a higher selectivity towards xylans, i.e. higher yields were obtained for xylo-oligosaccharides than for gluco-oligosaccharides in a proportion much above their original ratio in the pine nut shells hemicelluloses. Chemical bonds between xylose units in the xylan backbone as well as chemical bonds to arabinose were more easily cleaved than glucose and mannose bonds in glucomannans, as it is well known from the overall hydrolysis reactivity of the different hemicelluloses [34, 35].
The microwave-assisted autohydrolysis was less effective when compared to standard autohydrolysis, for which an equivalent maximal oligosaccharides yield was obtained for lower severity (log R0 4.01, 16.11 g/L) [18]. This suggests that autohydrolysis, either microwave-assisted or conventional, requires for optimal oligosaccharides production process severities between 4 and 4.5.
3.3 Microwave-assisted autohydrolysis under isothermal conditions
The microwave-assisted autohydrolysis was evaluated in more detail for process severities between 4 and 4.5, using an isothermal process at 190 °C with different durations of the isothermal periods from 0 to 60 min. The results are shown in Fig. 3. The best condition, with a xylo-oligosaccharides recovery of 34.64 g/100 g of the xylan in pine nut shells (concentration of 16.49 g/L), was obtained at log R0 4.2. This corresponds to a higher yield as compared to that obtained with a standard autohydrolysis of 28.74 g/100 g xylan [18], which was obtained with a log R0 4.01.
Composition (g/L) of the liquid fractions obtained after isothermal microwave-assisted autohydrolysis. as a function of log R0: A filled diamond, xylo- and mannan-oligosaccharides; filled triangle, arabino-oligosaccharides; filled square, gluco-oligosaccharides; B filled square, glucose; filled diamond, xylose and mannose; filled triangle, arabinose; C filled circle, acetic acid; filled triangle, formic acid; empty cirle, levulinic acid; filled diamond, furfural; filled square, 5-hydroxymethylfurfural. Lines are used for eye guidance only
For non-isothermal processes at comparable severity, microwave-assisted autohydrolysis seemed to show a lower performance than standard autohydrolysis, which could be explained by the fact that the ideal conditions have been exceeded. Precisely the isothermal conditions showed that in fact microwave treatment was more efficient than non-isothermal with standard methods.
Oligosaccharide hydrolysis to monosaccharides started simultaneously and slightly ahead of hemicellulose depolymerization to oligosaccharides. Xylose and mannose increased strongly in the autohydrolysis liquor from log R0 4.0 to 4.15 and remained constant subsequently until their decrease starting at log R0 4.47 (Fig. 3B). Arabinose content increased up to log R0 4.2 and decreased subsequently to zero at log R0 4.47. Glucose contents were rather stable with the different severities.
Monosaccharide degradation accompanied their presence in the autohydrolysis liquor, with a considerable increase of furan and aliphatic acids, therefore starting before the maximum oligosaccharides yield. Acetic acid started to accumulate from log R0 3.96 onwards, reaching 3.72 g/L. Furfural and HMF were found in low concentrations, higher for furfural (1.49 g/L) given the higher amounts of pentoses in the pine nutshell hemicelluloses and their higher dehydration susceptibility. Other sugar degradation by-products such as levulinic and formic acids were also present at low concentrations [36].
The total aliphatic acid concentration (including acetic acid) for the optimal xylo-oligosaccharide production condition was below 3 g/L, thereby not precluding, as potential microbial inhibitors, the possible future use of this hydrolysate for fermentation processes [36].
Overall, microwave-assisted autohydrolysis of pine nut shells allowed the production of oligosaccharides in values similar to those obtained with conventional autohydrolysis of the same material. This result was slightly different from studies performed by obtained by del Río et al. [37] that compared conventional and microwave-assisted autohydrolysis for the obtention of oligosaccharides from Paulownia obtaining the higher values of OS, with milder conditions with microwave in comparison to conventional water treatment. Taking into account the raw material composition and structure differences in operational and energy conditions of the autohydrolysis is a key factor for evaluating both processes.
3.4 Degree of polymerization of oligosaccharides
The characterization of the polymerization degree (DP) of the oligosaccharides obtained for the different severities of the isothermal autohydrolysis of pine nut shells was performed. The REZEX™ 195 RCM-Monosaccharide column and a malto-oligosaccharide standard including maltose, maltotriose, maltopentose and maltohexose with DPs of 2 to 6, respectively, were used to estimate the DP of the obtained pine nut shells oligosaccharides. With this column, the oligomers with higher DP have shorter retention time [38].
Figure 4 shows the chromatographic profiles of seven pine nut shell hydrolysates obtained at the different severity conditions as well as of the malto-oligosaccharide standard (standard OS). The standard profile clearly shows the separation of all five oligosaccharide standards from DP2 to DP6. The oligosaccharides of the seven hydrolysates included disaccharides (DP2), trisaccharides (DP3), tetrasaccharides (DP4), pentasaccharides (DP5) and hexasaccharides (DP6). The peak for the column retention time of approximately 45 min corresponds to xylose.
Depending on the autohydrolysis severity, different molecular weight distributions were found for the produced oligosaccharides. For the mildest autohydrolysis conditions, the obtained oligosaccharides were oligomers with 6 and above monomers. With increased severity, the oligosaccharide molecular weight progressively decreased, leading preferably to the accumulation of dimers and trimers.
Figure 5 shows the concentration profile of the various oligosaccharides with the increase in autohydrolysis severity. At the conditions leading to the highest concentration (log R0 4.2), the oligosaccharides were a mixture of oligomers with 2 to 5 units, with the highest proportion being dimers with a concentration of 3 g/L. Oligomers with DP4 start to increase after log R0 4.35, and are in the highest quantity at log R0 4.47, reaching a concentration of 4.1 g/L. DP6 oligomers reach the maximum value (1.4 g/L) at log R0 4.2. Monosaccharides measured in the RSO column (not shown in Fig. 5) follow the same trend as those measured in the Aminex 87H column, as previously discussed [39, 40].
The biological activity of xylo-oligosaccharides depends directly on their DP that typically ranges from 2 to 12. Xylo-oligosaccharides with DP ≤ 4 have prebiotic applications because they promote the propagation of beneficial bacteria in the intestinal tract, such as bifidobacteria, which inhibit the growth of pathogenic bacteria [41, 42]. Uronic acid–containing xylo-oligosaccahrides are known to present antioxidant and antialergic properties [42].
The pine nut shell–derived oligosaccharides by autohydrolysis have previously been tested and proved chemically stable in time, and for temperature and pH under relevant conditions for stomachal digestive conditions, thereby supporting their potential nutritional/bioactive properties [18]. The ability of these oligosaccharides to be processed and to pass the stomach undigested deems them as potential non-digestible oligosaccharides, a trait that should be further characterised and explored.
3.5 Composition of the solids
Within a biorefinery framework, it is necessary to envisage the full resource use, and therefore the biomass remaining after the microwave-assisted autohydrolysis has to be evaluated and valorised. Figure 6 shows the composition of the pine nut shells and of the solids obtained by autohydrolysis with the different hydrolysis severities and solid recovery. The values are given in mass proportion of each solid material.
The higher solid recovery of solids denotes the recalcitrance of the raw material related to the treatment. Only at severe conditions, the recovery decreased.
After autohydrolysis, the processed solids increase their glucan contents, due to the decrease of hemicelluloses that were solubilised to the liquor. The microwave-assisted process is selective for hemicellulose removal that increases with the severity of the treatment, and therefore the glucan proportion increased in the solids. This is consistent with previous results of biomass autohydrolysis, which were characterised to have a low effect on the cellulose and lignin fractions [30, 43].
The composition of the spent pine nut shells points out that they may be valorised by two pathways, either a biochemical route targeted to cellulose or a thermochemical route towards energy materials. Cellulose enzymatic hydrolysis was already tested with poor saccharification yields [18]. More severe chemical fractionation procedures are possibly necessary given that the lignin in pine nutshell is of the G type and therefore has low reactivity [1]. Current uses, namely as solid biofuel for combustion, are certainly possible, but other upgrading processes in the thermochemical platform of the biorefinery (e.g. biochar or bio-oil production) are of interest given that the biomass has a high heating value (HHV) favoured by the high lignin and low hemicelluloses content [43, 44]. In fact, most works of research carried out so far on pine nut shell valorisation have considered pyrolysis and biochar production [24, 45].
4 Conclusions
Pine nut shells are a good source for the production of oligosaccharides, namely xylo-oligosaccharides, which can be easily produced under environmentally friendly conditions using microwave-assisted autohydrolysis that allows a better performance by using milder operation conditions. The OS degree of polymerization varied from 6 to 2, being enriched with the highest DP and the lowest inhibitors at the best conditions. The spent solids are enriched in cellulose and can be further directed towards the biochemical or thermochemical platforms within the biorefinery framework.
Data availability
All data available in the manuscript.
References
Queirós CSGP, Cardoso S, Lourenço A, Ferreira J, Miranda I, Lourenço MJV, Pereira H (2019) Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers Biorefin 10(1):175–188. https://doi.org/10.1007/s13399-019-00424-2
Duarte LC, Esteves MP, Carvalheiro F, Girio FM (2007) Biotechnological valorization potential indicator for lignocellulosic materials. Biotechnol J 2(12):1556–1563. https://doi.org/10.1007/s13399-019-00424-2
Poletto P, Pereira GN, Monteiro CRM, Pereira MAF, Bordignon SE, de Oliveira D (2020) Xylooligosaccharides: transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process Biochem 91:352–363. https://doi.org/10.1016/j.procbio.2020.01.005
Pinales-Márquez CD, Rodríguez-Jasso RM, Araújo RG, Loredo-Treviño A, Nabarlatz D, Gullón B, Ruiz HA (2021) Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: a review. Ind Crop Prod 162:113274. https://doi.org/10.1016/j.indcrop.2021.113274
Jana UK, Suryawanshi RK, Prajapati BP, Kango N (2021) Prebiotic mannooligosaccharides: synthesis, characterization and bioactive properties. Food Chem 342:128328. https://doi.org/10.1016/j.foodchem.2020.128328
Puligundla P, Oh S-E, Mok C (2016) Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: a review. Carbon letters 17(1):1–10. https://doi.org/10.5714/cl.2016.17.1.001
Carvalheiro F, Duarte LC, Gírio F, Moniz P (2016) Hydrothermal/liquid hot water pretreatment (autohydrolysis): a multipurpose process for biomass upgrading. In: Mussatto SI (ed) Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery. Elsevier, Amsterdam, pp 315–347
Singh V, Tiwari A, Kumari P, Tiwari S (2006) Microwave-promoted hydrolysis of plant seed gums on alumina support. Carbohydr Res 341(13):2270–2274. https://doi.org/10.1016/j.carres.2006.05.021
Gude VG, Patil P, Martinez-Guerra E, Deng S, Nirmalakhandan N (2013) Microwave energy potential for biodiesel production. Sustain Chem Process 1(1):5. https://doi.org/10.1186/2043-7129-1-5
Kurtulbaş E, Sevgen S, Samli R, Şahin S (2022) Microwave-assisted extraction of bioactive components from peach waste: describing the bioactivity degradation by polynomial regression. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02909-z
Kehili M, Isci A, Thieme N, Kaltschmitt M, Zetzl C, Smirnova I (2022) Microwave-assisted deep eutectic solvent extraction of phenolics from defatted date seeds and its effect on solubilization of carbohydrates. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03027-6
Amarante SJ, Catarino MD, Marçal C, Silva AMS, Ferreira R, Cardoso SM (2020) Microwave-assisted extraction of phlorotannins from Fucus vesiculosus. Mar Drugs 18(11):559. https://doi.org/10.3390/md18110559
Kappe CO (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43(46):6250–6284. https://doi.org/10.1002/anie.200400655
de la Hoz A, Diaz-Ortiz A, Moreno A (2005) Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev 34(2):164–178. https://doi.org/10.1039/B411438H
Aggarwal NK, Kumar N, Mittal M (2022) Current trends in pretreatment technologies for bioethanol production: biorefinery concept. In: Aggarwal NK, Kumar N, Mittal M (eds) Bioethanol production: past and present. Springer International Publishing, Cham, pp 27–45
Chandel H, Kumar P, Chandel AK, Verma ML (2022) Biotechnological advances in biomass pretreatment for bio-renewable production through nanotechnological intervention. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02746-0
Warrand J, Janssen HG (2007) Controlled production of oligosaccharides from amylose by acid-hydrolysis under microwave treatment: comparison with conventional heating. Carbohydr Polym 69(2):353–362
Torrado I, Dionisio A, Fernandes MC, Roseiro LB, Carvalheiro F, Pereira H, Duarte LC (2023) Production of oligosaccharides from pine nut shells by autohydrolysis. Bioenergy Res. https://doi.org/10.1007/s12155-023-10585-8
Overend RP, Chornet E (1987) Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos Trans Royal Soc of London Series A-Math Phys Eng Sci A321(1561):523–536. https://doi.org/10.1098/rsta.1987.0029
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass, 2012th edn. National Renewable Energy Laboratory, Golden, Colorado
Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C. J.; Sluiter, J.; Templeton, D. (2008) Determination of sugars, byproducts, and degradation products in liquid fraction process samples; NREL/TP-510-42623; NREL
Vidal B, Dien B, Ting K, Singh V (2011) Influence of feedstock particle size on lignocellulose conversion: a review. Appl Biochem Biotechnol 164(8):1405–1421. https://doi.org/10.1007/s12010-011-9221-3
Da Silva Lacerda V, Pérez Lebeña E, Martín Ramos P, Hernández Navarro S, Sánchez Báscones M, Navas Gracia LM, Correa Guimaraes A, López Sotelo JB, Martín Gil J (2016) Efficient microwave-assisted acid hydrolysis of lignocellulosic materials into total reducing sugars in ionic liquids. Cellul Chem Technol 50(7-8):761–770
Chen D, Chen X, Sun J, Zheng Z, Fu K (2016) Pyrolysis polygeneration of pine nut shell: quality of pyrolysis products and study on the preparation of activated carbon from biochar. Bioresour Technol 216:629–636. https://doi.org/10.1016/j.biortech.2016.05.107
Jia J, Qu YC, Gao Y, Yuan YP, Wang KK, Yang F, Wu GF, Li YF (2013) Separation of lignin from pine-nut hull by the method of HBS and preparation of lignin-PEG-PAPI. Appl Mech Mater 320:429–434. https://doi.org/10.4028/www.scientific.net/AMM.320.429
Pereira H, Graça J, Rodrigues JC (2003) Wood chemistry in relation to quality. In: Wood quality and its biological basis, vol 35, Oxford, pp 53–86
Martins PL, Florez L, Lopez JE, Ferrer A, Duarte LC, Gírio F, Carvalheiro F (2014) Autohydrolysis as a sustainable alternative for the upgrade of sugarcane straw. Chempor, Porto, Portugal FEUP Editores
Moniz P, Gírio FM, Pereira H, Carvalheiro F (2009) Hydrothermal processing of corn residues: process optimisation and products characterisation, (2009) NWBC-2009. In: Kuokka-Ihalainen A (ed) The 2nd Nordic Wood Biorefinery Conference. VTT, pp 89–95
Silva-Fernandes T, Duarte LC, Carvalheiro F, Loureiro-Dias MC, Fonseca C, Girio F (2015) Hydrothermal pretreatment of several lignocellulosic mixtures containing wheat straw and two hardwood residues available in Southern Europe. Bioresour Technol 183:213–220. https://doi.org/10.1016/j.biortech.2015.01.059
Moniz P, Pereira H, Quilhó T, Carvalheiro F (2013) Characterisation and hydrothermal processing of corn straw towards the selective fractionation of hemicelluloses. Ind Crop Prod 50:145–153. https://doi.org/10.1016/j.indcrop.2013.06.037
Ho AL, Carvalheiro F, Duarte LC, Roseiro LB, Charalampopoulos D, Rastall RA (2014) Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresource Technology. 152, 526-9. bunch fibre by a non-isothermal process. Bioresour Technol 152:526–529. https://doi.org/10.1016/j.biortech.2013.10.114
Garrote G, Domínguez H, Parajó JC (2004) Production of substituted oligosaccharides by hydrolytic processing of barley husks. Ind Eng Chem Res 43(7):1608–1614. https://doi.org/10.1021/ie0342762
Parajó JC, Garrote G, Cruz JM, Dominguez H (2004) Production of xylooligosaccharides by autohydrolysis of lignocellulosic materials. Trends Food Sci Technol 15(3-4):115–120. https://doi.org/10.1016/j.tifs.2003.09.009
Moniz P, João L, Duarte LC, Roseiro LB, Boeriu CG, Pereira H, Carvalheiro F (2015) Fractionation of hemicelluloses and lignin from rice straw by combining autohydrolysis and optimised mild organosolv delignification. BioResources 10(2):2626–2641. https://doi.org/10.15376/biores.10.2.2626-2641
Ruiz HA, Cerqueira MA, Silva HD, Rodriguez-Jasso RM, Vicente AA, Teixeira JA (2013) Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohydr Polym 92(2):2154–2162. https://doi.org/10.1016/j.carbpol.2012.11.054
del Río PG, Pérez-Pérez A, Garrote G, Gullón B (2022) Manufacturing of hemicellulosic oligosaccharides from fast-growing Paulownia wood via autohydrolysis: microwave versus conventional heating. Ind Crop Prod 187:115313. https://doi.org/10.1016/j.indcrop.2022.115313
Liu Z, Fels M, Dragone G, Mussatto SI (2021) Effects of inhibitory compounds derived from lignocellulosic biomass on the growth of the wild-type and evolved oleaginous yeast Rhodosporidium toruloides. Ind Crop Prod 170:113799. https://doi.org/10.1016/j.indcrop.2021.113799
Jurková M, Čejka P, Štěrba K, Olšovská J (2014) Determination of total carbohydrate content in beer using Its pre-column enzymatic cleavage and HPLC-RI. Food Anal Methods 7(8):1677–1686. https://doi.org/10.1007/s12161-014-9805-y
Díaz S, Ortega Z, Benítez AN, Costa D, Carvalheiro F, Fernandes MC, Duarte LC (2021) Assessment of the effect of autohydrolysis treatment in banana’s pseudostem pulp. Waste Manag 119:306–314. https://doi.org/10.1016/j.wasman.2020.09.034
Moniz P, Ho AL, Duarte LC, Kolida S, Rastall RA, Pereira H, Carvalheiro F (2016) Assessment of the bifidogenic effect of substituted xylo-oligosaccharides obtained from corn straw. Carbohydr Polym 136:466–473. https://doi.org/10.1016/j.carbpol.2015.09.046
de Freitas C, Carmona E, Brienzo M (2019) Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact Carbohydr Diet Fibre 18:100184. https://doi.org/10.1016/j.bcdf.2019.100184
Chen Y, Xie Y, Ajuwon KM, Zhong R, Li T, Chen L, Zhang H, Beckers Y, Everaert N (2021) Xylo-Oligosaccharides, preparation and application to human and animal health: a review. Front Nutr 8. https://doi.org/10.3389/fnut.2021.731930
Nabarlatz D, Ebringerová A, Montané D (2007) Autohydrolysis of agricultural by-products for the production of xylo-oligosaccharides. Carbohydr Polym 69(1):20–28. https://doi.org/10.1016/j.carbpol.2006.08.020
Álvarez A, Pizarro C, García R, Bueno JL (2015) Spanish biofuels heating value estimation based on structural analysis. Ind Crop Prod 77:983–991. https://doi.org/10.1016/j.indcrop.2015.09.078
Qin L, Wu Y, Hou Z, Jiang E (2020) Influence of biomass components, temperature and pressure on the pyrolysis behavior and biochar properties of pine nut shells. Bioresour Technol 313:123682. https://doi.org/10.1016/j.biortech.2020.123682
Acknowledgements
The authors acknowledge the pine nut processing mill Preparadora de Pinhões Lda. (Pegões, Montijo, Portugal) for their kind supply of the pine nut shells. Thanks are due to the School of Agriculture (Department of Technology and Applied Sciences), Polytechnic Institute of Beja, for the use of the microwave equipment (ALENT-07-0262-FEDER-001870).
Funding
Open access funding provided by FCT|FCCN (b-on). The work was supported by the project ALT20-03-0145-FEDER-000034 funded by Alentejo2020 Program through the European Regional Development Fund (ERDF), and by Fundação para a Ciência e a Tecnologia (FCT) through projects UID/AGR/00239/2020, UIDB/05183/2020 and LA/P/0121/2020 supporting Centro de Estudos Florestais, MED, and CHANGE respectively. Ivone Torrado received the PhD grant from FCT within the SUSFOR doctoral programme (PD/BD/114175/2016). Maria C Fernandes received funding from the project Procurement of Highly Qualified Human Resources (ALT20-05-3559-ESF-000076) Alentejo2020 Program through the European Social Fund.
Author information
Authors and Affiliations
Contributions
Conceptualization: Luís C. Duarte, Ivone Torrado, Maria C. Fernandes. Methodology: Ivone Torrado, Beatriz Guapo Neves. Formal analysis and investigation: Luís C. Duarte, Ivone Torrado. Writing: Ivone Torrado. Preparation: Luís C. Duarte, Ivone Torrado. Writing—review and editing: Ivone Torrado, Luís C. Duarte, Helena Pereira, Florbela Carvalheiro, Maria C. Fernandes. Funding acquisition: Maria C. Fernandes. Resources: Maria C. Fernandes. Supervision: Luís C. Duarte, Helena Pereira, Maria C. Fernandes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent for publication
All authors read and approved the final manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Pine nut shell was subjected for the first time to microwave-assisted (MW) autohydrolysis.
• MW autohydrolysis process is highly selective for hemicellulose removal.
• Hydrolysates contained mixture of oligomeric compounds, mainly xylo-oligosaccharides.
• Mild operation conditions (190 °C, 30 min) produced 18.48 g/L of oligosaccharides.
• Oligosaccharide polymerization degree varied from 2 to 6.
Supplementary information
ESM 1
Figure S1-Temperature profiles for the MW-assisted autohydrolysis processes for non-isothermal (A) and isothermal processes (B). (DOCX 33 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torrado, I., Neves, B.G., da Conceição Fernandes, M. et al. Microwave-assisted hydrothermal processing of pine nut shells for oligosaccharide production. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-023-05244-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05244-z